Abstract
Aims: The present study was designed to examine the five-year angiographic follow-up of MACE-free patients enrolled in the PRISON II study.
Methods and results: In the PRISON II study a total of 200 patients were randomised to either bare metal stents (BMS) or sirolimus-eluting stents (SES) after successful recanalisation of total coronary occlusions (TCO). Patients free of MACE with available angiography at six months were approached for repeated angiography at five years. The primary endpoint was in-stent very late luminal loss (VLLL) at five years. The secondary endpoint was additional late luminal loss (ALLL) between six months and five years. At five years, repeated angiography was performed in 72 patients, 50/82 (61%) in the SES group and 22/58 (38%) in the BMS group. In-stent VLLL was lower in the SES group (0.19 mm±0.72 vs. 0.51 mm±0.71, p=0.09) compared to the BMS group and in-segment VLLL was comparable in both groups (0.01 mm±0.58 vs. 0.03 mm±0.73, p=0.89). Late catch-up in lumen diameter was observed in the SES group with a trend towards increased ALLL compared to the BMS group (in-stent, 0.35 mm±0.88 vs. 0.04 mm±0.81, p=0.16; in-segment, 0.20 mm±0.74 vs. -0.05 mm±0.73, p=0.19).
Conclusions: At five-year angiographic follow-up, late catch-up was observed after successful recanalisation of TCOs treated with SES. Despite a late catch-up, the angiographic results of SES were superior in-stent and similar in-segment compared to BMS.
Abbreviations
ALLL: additional late luminal loss
BMS: bare metal stent
DES: drug-eluting stent
LLL: late luminal loss
MACE: major adverse cardiac events
MLD: minimal lumen diameter
PCI: percutaneous coronary intervention
PES: paclitaxel-eluting stent
SES: sirolimus-eluting stent
TCO: total coronary occlusion
VLLL: very late luminal loss
Introduction
Percutaneous coronary intervention (PCI) of total coronary occlusions (TCO) remains a major challenge for interventional cardiologists. Successful recanalisation of TCOs can alleviate angina, improve left ventricular function, reduce the need for coronary artery bypass surgery and increase long-term survival1,2. TCOs represent complex lesions at high risk for development of restenosis after balloon angioplasty or bare metal stent (BMS) implantation3-6. The introduction of drug-eluting stents (DES) was associated with a landmark reduction of restenosis7,8. Characterised by long lesions and small luminal diameters, TCOs seem to benefit particularly from the antiproliferative properties of DES9,10. In the PRISON II trial, we compared sirolimus-eluting stents (SES) and BMS in patients with TCO and showed a clear reduction in binary restenosis in favour of the SES group. At six-month angiography, intima hyperplasia was eliminated11. As a consequence, clinical outcome was superior at one-year follow-up with a strongly reduced rate of target lesion revascularisation (TLR). At long-term follow-up, clinical outcome was still in favour of DES in occlusive and non-occlusive lesions12-17. However, concerns have emerged about a so-called “late catch-up phenomenon” in DES after observing delayed neointima hyperplasia in a porcine model18. In addition, a first report of late “catch-up” after DES implantation in human was published19. Delayed neointima stent strut coverage, chronic inflammation and hypersensitivity reaction on the permanent polymers were suggested as possible mechanisms of late luminal rebound in DES20-22. Although late catch-up with DES in non-occlusive lesions was recognised, long-term angiographic outcome in complex lesions like TCO is currently unknown.
Aim of the study
The present study was designed to evaluate the staged long-term luminal response pattern after BMS or SES implantation for TCO in patients free of major adverse cardiac events (MACE) during five-year follow-up.
Methods
SOURCE POPULATION
Between January 2003 and September 2004 a total of 200 patients were included in the PRISON II trial in two high-volume PCI hospitals in the Netherlands (Onze Lieve Vrouwe Gasthuis, Amsterdam, and St. Antonius Hospital, Nieuwegein). A detailed description of the PRISON II study design has previously been published11. In brief, patients with a totally occluded native coronary artery of at least two weeks and evidence of ischaemia in the area of the target vessel were randomised to either a conventional BMS (BX VELOCITYTM; Cordis, Johnson & Johnson, Warren, NJ, USA) or a SES (CYPHER®; Cordis, Johnson & Johnson, Warren, NJ, USA). Patients were excluded if the lesion could not be crossed with a guidewire, in case of severe renal failure, defined as creatinine >130 mmol/L (or >1.47 mg/dl) or the inability to provide written informed consent. After successfully crossing the lesion with a guidewire, patients were randomised using an independent telephone allocation service. Prior to the index angiography, patients were pretreated with a loading dose, at least one day before the procedure, of 300 mg of clopidogrel and 80 mg of aspirin. After the index procedure, patients received 80 mg aspirin once daily indefinitely and 75 mg of clopidogrel daily for at least six months. Coronary angiograms were performed at baseline, immediately after the index procedure and at six-month follow-up. Clinical follow-up was obtained at 30 days, six months and at one, two, three, four, and five-year follow-up. All clinical results have been published previously11,16,17.
STUDY DESIGN
For the composition of the current study cohort, all patients from the original PRISON II trial with available six-month angiography were sought for repeated angiography. Patients free of MACE were approached for repeat angiography at five-year follow-up. MACE was defined as a composite of target lesion revascularisation (TLR), myocardial infarction or death. Exclusion criteria were severe renal failure or the inability to provide written informed consent. Standard coronary angiography techniques were used from either the radial or femoral artery with a heparin bolus prior to the procedure. The institutional ethics committee of both hospitals approved the study protocol and all patients included gave written informed consent prior to repeat angiography.
STUDY ENDPOINTS
The primary endpoint was in-stent very late luminal loss (VLLL), defined as the difference between five-year follow-up and post-PCI minimal lumen diameter (MLD). The secondary endpoint was in-stent additional late luminal loss (ALLL), defined as the difference between the VLLL and late luminal loss (LLL) at six months (LLL was defined as the difference between six-month and post-PCI MLD). Other secondary endpoints included in-stent MLD and percentage of diameter stenosis at five-year follow-up.
QUANTITATIVE CORONARY ANGIOGRAPHY AND DEFINITIONS
Angiograms were digitally recorded and assessed offline at the independent quantitative angiographic core laboratory of the St. Antonius Hospital Nieuwegein with an automatic edge detection system (CMS version 5.3; Medis Medical Imaging Systems, Leiden, The Netherlands) by experienced co-workers who were not provided with any clinical information on the type of stent used. The non-tapered tip of the catheter was used as the calibration standard. Following intracoronary injection of nitroglycerine (100-200 µg), all lesions were assessed in the same two near-orthogonal views that were used at inclusion in the PRISON II trial. The projection showing the smallest diameter (worst view) was used for quantitative coronary angiography analysis, and views with the least foreshortening were used for measuring the length of the occlusion. Quantitative coronary analysis was used to evaluate the stented area (in-stent) and the area that included the stented segment as well as the 5 mm margins proximal and distal to the stent (in-segment). Quantitative measurement included reference diameter of the vessel and the in-stent and in-segment MLD. The reference diameter was measured in the disease-free target vessel segment. Additional study parameters in the present study were calculated from the acquired parameters.
Statistics
Differences in baseline and outcome variables between the BMS and DES group were assessed using the Student’s t or Mann-Whitney test where appropriate for continuous variables and Fisher’s exact test for categorical variables. For within group comparisons we used the paired Student’s t-test for continuous variables and McNemar’s test for categorical variables. No formal statistical method was used to adjust for multiple comparisons. All computations were performed in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
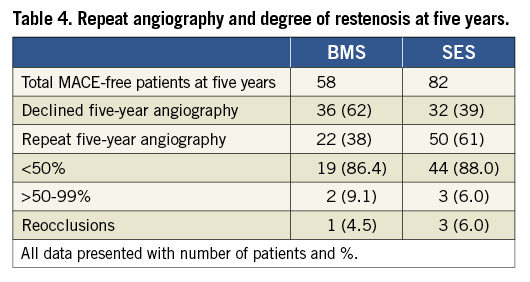
A total of 200 patients with TCO were randomised to either BMS or SES (Figure 1). Of these, six patients in both groups refused angiographic follow-up at six months, none of them had symptoms and no adverse events occurred. At six-month follow-up, 188 patients (BMS 94, SES 94) underwent repeat angiography. During five-year follow-up 48 patients (BMS 36/94 [38%], SES 12/94 [13%]) experienced MACE. As a consequence 140 patients (BMS 58/94 [72%], SES 82/94 [87%]) were eligible for five-year repeat angiography. Of these MACE-free patients, 68 patients (BMS 36/58 [62%], SES 32/82 [39%]) declined the repeat angiogram. Again, these patients were absent of symptoms and free of adverse events. The final study population involved 72 patients, 22/58 (38%) in the BMS group and 50/82 (61%) in the SES group. There were no significant differences in baseline characteristics between patients who underwent or declined the five-year repeat angiography (data not shown).
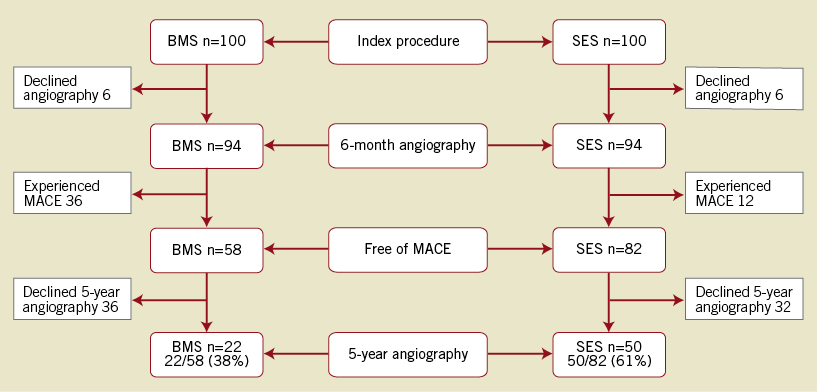
Figure 1. Five-year angiography in MACE-free patients. BMS: bare metal stent; SES: sirolimus-eluting stent
Overall, both groups were matched for baseline clinical and angiographic characteristics. In the SES group, multivessel disease was more common, the occlusion length was longer and the maximum balloon size smaller compared to the BMS group (55.1% vs. 36.4%; p=0.02; 21.84±9.6 vs. 17.14±5.68, p=0.04; 3.45±0.27 vs. 3.23±0.27, p=0.02) (Table 1 and Table 2). The median interval of angiographic follow-up was comparable between both groups at six months (BMS, 6.0 months, interquartile range [IQ] 5.9-6.6 vs. SES 6.1 months, IQ 5.9-6.3; p=0.87) and five years (BMS, 4.9 years, IQ 4.6-5.3 vs. SES, 5.0 years, IQ 4.5-5.4; p=0.98).
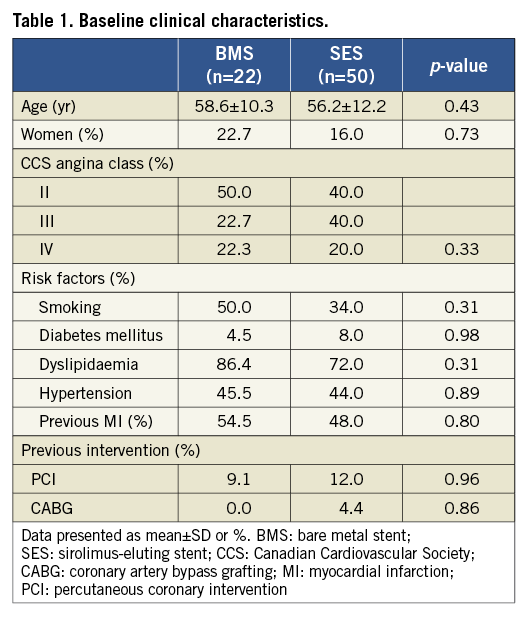
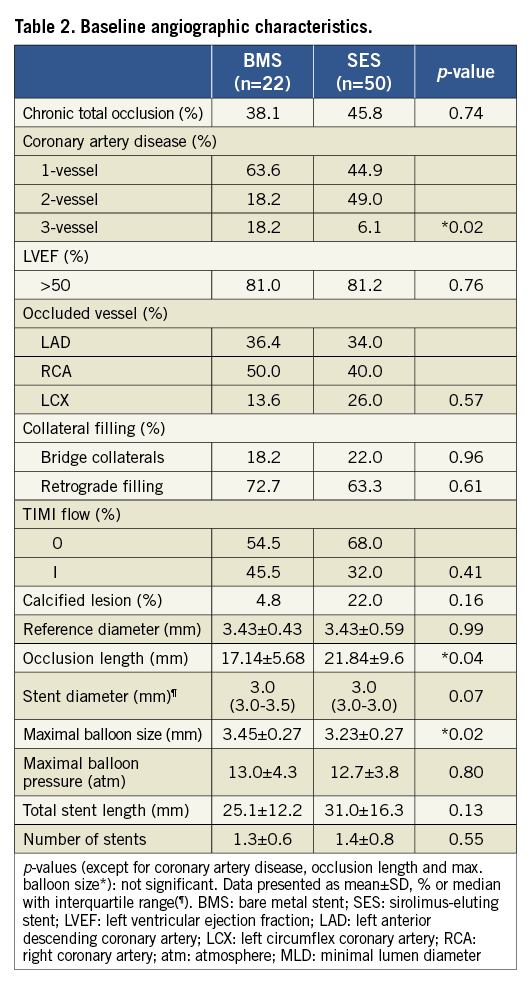
Table 3 summarises the angiographic results. After the index PCI the MLD was comparable between both stenting groups. At six-month follow-up the in-stent MLD was larger and a trend was apparent towards larger in-segment MLD in the SES group. As a consequence, LLL was in favour of the SES group.
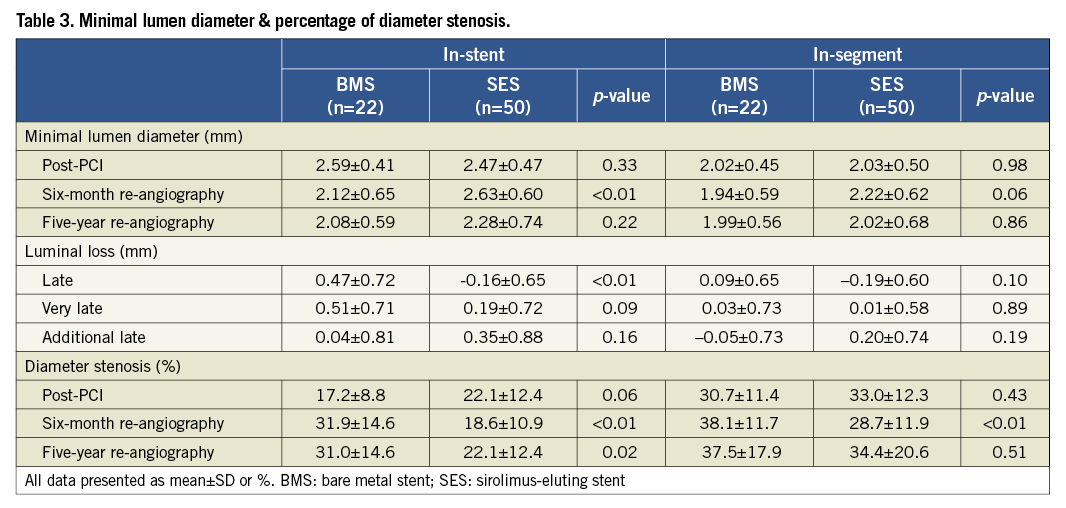
At five-year angiographic follow-up the primary endpoint in-stent VLLL remained nearly significant though reduced in favour of the SES group (0.19 mm±0.72 vs. 0.51 mm±0.71, p=0.09) with comparable in-segment results (in-segment 0.01±0.58 vs. 0.03± 0.73, p=0.89). However, the MLD decreased between six months and five years in the SES group, whereas it remained constant in the BMS group. As a result, a trend was demonstrated towards an increased ALLL in the SES group (in-stent, 0.35 mm±0.88 vs. 0.04 mm±0.81, p=0.16; in-segment, 0.20±0.74 vs. –0.05±0.73; p=0.19). The distribution histogram of in-stent ALLL for both stenting groups is shown in Figure 2. The percentage of diameter stenosis showed similar results at six months and five years (Figure 3) with a significantly reduced in-stent and similar in-segment percentage of diameter stenosis at five years (in-stent, 22.1%±12.4 vs. 31.0%±14.6, p=0.02; in-segment, 34.4%±20.6 vs. 37.5%±17.9; p= 0.51). Finally, the percentage of occluded stents, restenosed (>50%) stents and non-restenosed (<50%) stents was similar between both groups at five years (Table 4).
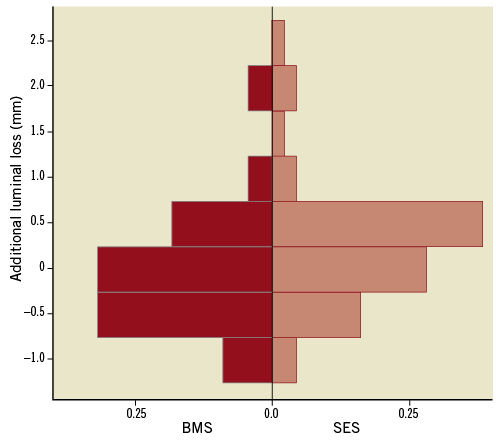
Figure 2. In-stent additional late luminal loss. BMS: bare metal stent; SES: sirolimus-eluting stent
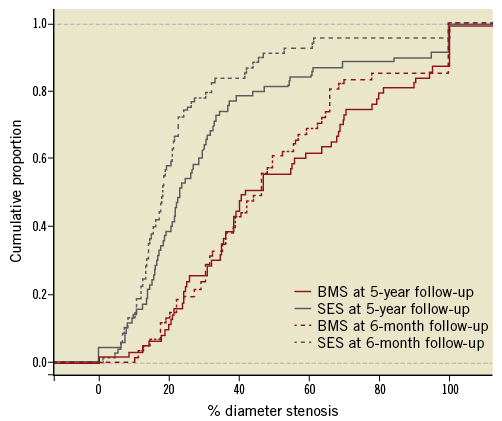
Figure 3. Cumulative proportion of in-stent diameter stenosis. BMS: bare metal stent; SES: sirolimus-eluting stent
Discussion
In the present study, a “late catch-up phenomenon” was observed in TCOs treated with SES. At five-year repeat angiography, in-stent and in-segment additional late lumen loss was 0.35±0.88 mm and 0.20±0.74 mm in the SES group compared to 0.04±0.81 mm and -0.05±0.73 in the BMS group. Despite late catch-up in lumen diameter being observed in the SES group, results were superior in-stent with a reduced VLLL compared to the BMS group. In-segment, the observed late luminal rebound in the SES group resulted in similar VLLL between both stenting groups.
In the current analysis, we showed comparable luminal diameters in the BMS group at six-month and five-year follow-up. Long-term serial angiography in the historical BMS era showed a triphasic luminal response after stent implantation. Excessive proliferation of smooth muscle cells and extracellular matrix caused a maximum peak in neointima hyperplasia between six and nine months23,24. Thereafter, neointima regressed due to fibrotic scar formation. Matured smooth muscle cells and matrix proteoglycans in the neointima were reduced up to a period of four years. Beyond this point, gradual very late luminal renarrowing was observed until 11-year angiographic follow-up. Pathologic examinations revealed in-stent neoatherosclerosis as the main driver of very late luminal renarrowing. Beyond five years, histologic analysis showed evidence of chronic inflammation around the stent struts with heavy infiltration of lipid-laden macrophages25. In our TCOs population, long-term angiographic results were consistent with other previous findings after BMS implantation in non-occlusive lesions.
In the SES group, we found a clear “late catch-up phenomenon” at five-year follow-up. Sousa et al were the first to describe long-term serial angiographic data after SES in a small sample study with simple de novo lesions26. Angiographic follow-up was performed at one year, two and four years after stent implantation. At two-year follow-up, late luminal loss was –0.19±0.15 mm, whereas late luminal rebound was observed after four years with final luminal loss of 0.09±0.23 mm. These observations in SES were confirmed with intravascular ultrasound27,28. Aoki et al observed an increase in neointimal volume from 0 to 8.4±5.8 mm3 (p<0.001) at four years, although no significant change occurred between two and four years, 7.0±6.7 mm3 vs. 8.4±5.8 mm3 (p=0.25)27. More recently, mild late catch-up in lumen diameter was confirmed in larger cohort studies in both SES and paclitaxel-eluting stents (PES)29-31. At two-year follow-up, delayed in-stent luminal loss was 0.17±0.50 mm and 0.20±0.63 mm in SES compared to 0.13±0.50 mm in PES. Yang et al reported the four-year angiographic follow-up in 195 patients treated with SES. In-stent luminal loss at 7.1 months was 0.15±0.39 mm and 0.34±0.55 mm at 45.9 months32. The SIRTAX LATE trial was the only randomised trial with five-year angiographic follow-up comparing SES versus PES33. They obtained five-year angiographic follow-up in approximately 43.8% of the 1,012 all-comer patients. The population consisted of high-risk patients with approximately 50% acute coronary syndromes and 60% multivessel disease. At five years, delayed luminal loss amounted to 0.37±0.73 mm for SES and 0.29±0.59 mm for PES. In our population of complex lesions, the presence of late catch-up (0.35±0.88 mm) after SES was more prominent compared to other observations at two to three years in de novo lesions. However, they were similar to the results of SIRTAX LATE, also representing a high-risk population. The main origin of the observed late luminal rebound remains largely hypothetical. Earlier reports postulated hypersensitivity reactions to the permanent polymers of DES as the main contributor to late luminal rebound20,21. However, recent autopsy examination identified early in-stent neoatherosclerosis as a possible mechanism of late “catch-up” after DES implantation, which was observed in a relatively early phase (median 430 days) as compared to BMS (median 2,160 days)34. It was hypothesised that delayed and incomplete stent strut coverage, which persists after DES up to 20% at two years, was the initiating mechanism of early in-stent neoatherosclerosis35. The inability to maintain a fully functional endothelialised luminal surface after DES may induce an extra influx of circulating lipids. Thus, an optimal environment was created for the early development of in-stent neoatherosclerosis. In the current study, no histological evidence was obtained to confirm the suggested gradual formation of early in-stent neoatherosclerosis.
Clinical implications
In this select group of MACE-free patients, we observed late catch-up in lumen diameter in the SES group. However, the recently published long-term clinical results of the PRISON II cohort showed no evidence for incremental clinical events17. There was a strong reduction in target lesion revascularisation at one year with parallel running survival curves until five-year follow-up. Apparently, therefore, the mild angiographic “late catch-up” in SES does not translate to more clinical events. These observations are in line with other long-term clinical observations in TCO13.
Limitations
The final study population involved only 22/58 (38%) of the MACE-free patients in the BMS group and 50/82 (61%) in the SES group. Since the baseline characteristics were similar between patients who underwent or declined the five-year repeat angiography, there are no reasons to assume that results would have been different if all patients had undergone repeat angiography at five years. In the BMS group more patients experienced MACE compared to the SES group. As a result, the population treated with SES, by definition, is different from that after BMS treatment. Therefore, comparison of late catch-up between both stenting groups should be interpreted cautiously. Finally, most observed differences between both stent groups did not reach statistical significance at a conventional level. This was attributed to the small sample size in the BMS group. However, the observed changes in MLD between both stent groups were comparable with other trials in all types of lesion30-33.
Conclusion
At five-year follow-up, angiographic late catch-up in lumen diameter was observed after successful recanalisation of TCO using SES compared to BMS. The in-stent MLD in the SES group was superior compared to the BMS group, whereas in-segment MLD was similar between both stent groups. Although late catch-up was present after SES implantation, this did not translate to more major adverse cardiac events compared to BMS. Long-term clinical results were still in favour of SES.
Funding
The PRISON II main study was supported by an unrestricted grant from the Cordis Corporation. The current study was executed without any financial support.
Conflict of interest statement
The authors have no conflicts of interest to declare.

