Abstract
Aims: The aim of this study was to investigate the angiographic and intravascular ultrasound (IVUS) findings of the Endeavor™ zotarolimus-eluting stent (ZES) in patients from a “real-world” clinical practice.
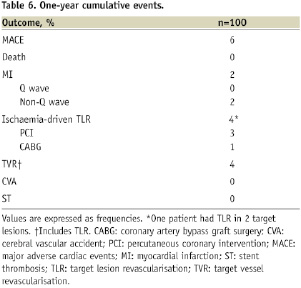
Methods and results: From January to March 2006, 100 patients undergoing routine or emergency percutaneous intervention were prospectively enrolled at one institution. Overall, 39% of the patients were diabetics and 80.8% of lesions were type B2/C. A total of 140 lesions were successfully treated with 174 ZES, and procedural success was 98%. Mean vessel diameter was 2.69 mm and mean lesion length was 16.0 mm; at 6-month angiographic follow-up (completed in 96%), in-stent late lumen loss was 0.66 mm, and in-segment restenosis was 8.2%. Angiographic restenosis was increased among diabetics (15.5 vs. 2.6%, p=0.009), and diabetes was the only significant predictor of angiographic restenosis (OR=15.27 [95%CI 2.45-95.04], p=0.003). By IVUS (performed in 88% at 6-month), % volume obstruction was 14.4±13.4%, and there was no late acquired incomplete stent apposition (ISA). At 1-year, overall MACE rate was 6%, including 5 TLRs (4% of patients), with no occurrence of stent thrombosis.
Conclusions: In this prospective “real-world” experience, the ZES demonstrated favourable angiographic and IVUS results in complex patients, with overall in-stent late lumen loss of 0.66 mm, and absence of late acquired ISA. At 1-year, there were no safety concerns including absence of death and stent thrombosis.
Introduction
Despite the overall marked efficacy of first generation drug-eluting stents (DES) on reducing restenosis, and therefore, the need for repeat lesion revascularisation as compared to bare metal stents (BMS),1,2 persistent concerns regarding deliverability, efficacy and long-term safety – especially in more complex subsets3-6 – has led to further development of DES technologies. These developments include novel antiproliferative drugs with alternative stent platforms and biocompatible drug carrier systems.7
Zotarolimus – a sirolimus analogue, is a recently developed pharmacologic agent with both antiproliferative and anti-inflammatory properties. The Endeavor™ DES system (Medtronic Vascular, Santa Rosa, CA, USA) technology incorporates: 1) zotarolimus; 2) a very low-profile cobalt alloy stent platform; and 3) a biocompatible drug carrier system, phosphorylcholines (PC).8 In the first-in-human (non-randomised) evaluation of the Endeavor zotarolimus-eluting stent (ZES), single non-complex lesions were successfully treated and late outcomes at 12 months demonstrated only a 2% rate for major adverse events with restenosis at 5.4%.7 The ZES also demonstrated similar performance in the Endeavor II and III trials, including overall in-stent restenosis rates <10% and very low rates of target lesion revascularisation (TLR) at nine months follow-up (~4-6%).9,10 Importantly, all studies showed sustained safety of the ZES during the long-term clinical follow-up, with no cases of very late stent thrombosis (>1 year) reported to date.7-12
However, the performance and impact of the ZES in the “real-world” is yet to be determined. We, therefore, performed a study to examine the 6-month angiographic and intravascular (IVUS) findings of the ZES in non-selected patients treated in a “real-world” clinical practice, and their relation to the 1-year clinical outcomes.
Methods
Study design and population
Between January and March 2006, 100 patients were enrolled in this prospective, non-randomised registry for percutaneous treatment with the ZES at one institution: Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil. Inclusion criteria were: 1) patients undergoing routine or emergency percutaneous coronary intervention with >18 years of age and 2) presence of at least one documented stenosis >50% in a native coronary vessel suitable for percutaneous coronary intervention (PCI) with stent implantation. During the enrolment interval, no patients were treated with other DES. There were no protocol prespecified limitations regarding the number of lesions and/or vessels that could to be treated with the study device.
The study complied with the Declaration of Helsinki regarding investigation in humans and was approved by the institutional Ethics Committee at the referred clinical institution. All patients provided written informed consent prior to procedure.
Device description
The ZES device has been described in detail elsewhere.8 In brief, the ZES incorporates the approved Driver™ stent (Medtronic Vascular, Santa Rosa, CA, USA) platform, a thin (0.0036-inch) chromium-cobalt-nickel based alloy which is designed to be stronger and denser than the conventional 316L stainless steel used in other stent platforms. The ZES’s thinner struts also result in a lower profile and improved deliverability, especially when targeting “distal” coronary segments or navigating through tortuous and calcified vessels, without compromising radial strength and visibility.13
Zotarolimus (formerly ABT-578) is a sirolimus-analogue compound with potent anti-inflammatory and antiproliferative properties. Its mechanism of action includes binding to the intracellular kinase binding protein (FKBP-12) inhibiting mammalian target of rapamycin (m-TOR) enzyme causing interruption of the G-1 phase of the cell cycle (cytostatic effect), therefore, preventing neointimal proliferation. Importantly, zotarolimus incorporates an unique tetrazole ring on its chemical structure that confers a >2-fold higher lipophilicity compared to sirolimus. Zotarolimus is coated onto the Driver stent platform using a biocompatible and non-thrombogenic phosphorylcholine polymer coating,14,15 in a dosage of 10 µg per mm of stent length. Preclinical studies with the ZES had demonstrated that ~95% of zotarolimus is released within 15 days after implantation, with the remaining drug absorbed into local cells by 30 days.8
Stenting procedure
All interventions were performed according to the current standard guidelines, and final procedure strategy was left upon operators’ discretion. The ZES device was available in two sets of measures: 8, 12, 14, 18, 24 and 30 mm in length for diameters of 2.25, 2.50, and 2.75 mm; and in lengths of 9, 12, 15, 18, 24 and 30 mm for diameters of 3.0, 3.5 and 4.0 mm. Multiple stenting procedure with the study device was allowed.
Dual antiplatelet therapy, including aspirin (100-325 mg daily) and clopidogrel (300 mg loading dose plus 75 mg daily), was started at least 24 hours before the procedure (commonly in elective patients); in case of emergency, a loading dose of 600 mg was given prior to procedure. Postprocedural aspirin was continued indefinitely and clopidogrel was maintained for only three months, according to the institution’s protocol for the Endeavor DES. During the procedure, intravenous heparin (70-100 units/Kg) was administered after sheath insertion to maintain an ACT >250 seconds (>200 seconds if glycoprotein IIb/IIIa inhibitors were used).
A routine 12-lead electrocardiogram was obtained a three time-points: before the procedure, immediately afterwards and 24-hours later. Blood sample laboratory analysis included CK and CK-MB before procedure (<24 hours), 18-24 hours after treatment and daily thereafter until hospital discharge.
Angiographic and IVUS analysis
After intracoronary nitrate administration (100-200 µg), serial coronary angiography was obtained at baseline, postprocedural and follow-up. Off-line quantitative coronary angiography (QCA) analysis was performed using the CMS-GFT™ system version 5.1 (Medis, Leiden, The Netherlands). The accuracy of the method has been previously reported.16 The minimum lumen diameter (MLD) and the mean reference diameter (RD), obtained from averaging 5 mm segments proximal and distal to the target lesion location, were used to calculate the diameter stenosis (DS=[1-MLD/RDx100]). Acute gain was the change in MLD from baseline to post-stent implantation; late lumen loss (LLL) was the change in MLD from the post-stent implantation angiogram to follow-up. Binary restenosis was defined as stenosis ≥50% at follow-up angiographic study, and was classified as focal (<10 mm) or diffuse (≥10 mm). QCA measurements were performed: 1) “in-stent”, within the stented segment and 2) “in-segment”, spanning the stented segment plus the 5 mm proximal and distal peri-stent areas.
Serial IVUS studies at postprocedural and follow-up were also performed after intracoronary nitrate administration (100-200 µg) with a motorised transducer pullback system (0.5 mm/second) and commercial scanners (CVIS and Galaxy 2, Boston Scientific Corporation, Natick, MA, USA) consisting of a rotating 40 MHz transducer with a 2.6 Fr imaging sheath. The imaging catheter was advanced approximately 10 mm beyond the distal end of the stented, and imaging acquisition continued through the proximal vessel. All IVUS images were recorded on 0.5-inch high-quality VHS videotapes or CD/DVD for off-line analysis. All IVUS images were digitised to perform qualitative and quantitative analysis according to the criteria of the ACC Clinical Expert Consensus.17 A coronary segment starting at the distal stented segment edge and extending to its proximal edge was analysed. A computer-based contour detection program was used for automated 3-dimensional reconstruction of the stented segment (Echoplaque, Indec Systems, Inc, Mountain View, CA, USA). Cross-sectional area measurements every 0.5 mm included lumen, stent, and external elastic membrane. Vessel volume, stent volume and lumen volume were calculated according to the Simpson’s rule. The in-stent neointimal volume was calculated as stent volume minus lumen volume. The percentage of stent obstruction (%NV) was calculated as neointimal volume divided by stent volume x 100; the plaque volume behind the stent (vessel volume minus stent volume) was also reported.
All cineangiograms and IVUS images were analysed at the Instituto Dante Pazzanese de Cardiologia’s Angiographic and Imaging Core Laboratories (São Paulo, Brazil) by experienced senior operators blinded to procedural data.
Endpoints, definitions and clinical follow-up
The study’s primary objectives were: in-stent late lumen loss by QCA; % volume obstruction and rates of incomplete stent apposition by IVUS at 6-month follow-up. The secondary objective was the occurrence of major adverse cardiac events (MACE) at 1-year clinical follow-up. MACE was defined as death, non-fatal myocardial infarction (MI), and target vessel revascularisation (TVR). Target vessel revascularisation included TLR, which was indicated in case of angiographic restenosis plus presence of symptoms and/or positive functional test for ischaemia. All deaths were considered to be cardiac unless a non-cardiac origin could be clearly established by clinical and/or pathological study. The diagnosis of MI was based on either the development of new pathological Q waves in ≥2 contiguous electrocardiogram leads and/or elevation of CK or its MB isoenzyme >3 times the upper normal limit postprocedural during index hospitalisation, or cardiac enzyme elevation >2 times the upper normal limit thereafter. Other endpoints included: angiographic restenosis and LLL by QCA; % NV by IVUS at follow-up; the incidence of stent thrombosis (ST) up to
1-year follow-up. ST was defined according to definitions proposed by the ARC.18
Angiographic success was defined as attainment of <20% residual stenosis by QCA in the treated segment post-ZES implantation. Procedural success was defined as angiographic success plus absence of MACE during hospitalisation. All patients were assigned for angiographic (and IVUS) re-evaluation at 6-month and
36-month follow-up. Clinical follow-ups consisted of medical visits, and were scheduled at 1, 6 and 12 months, and yearly up to three years after stent implantation. In this analysis, we report the
6-month angiographic and IVUS results, and clinical outcomes up to 12-month follow-up.
Statistical analysis
Data are presented as mean±1 standard deviation or frequencies. Statistical analysis was performed using SPSS software version 11.0 (Chicago, IL, USA). For comparisons of continuous variables, a 2-tailed, unpaired Student t test was used. Categorical data were compared using the Chi-square test or Fisher’s exact test. Multivariate analysis, with consideration of all variables with a value of P<0.10, were performed to identify independent predictors of angiographic binary restenosis. One year event-free survival for MACE was demonstrated by Kaplan-Meier curve. A value of p<0.05 was considered significant.
Results
Baseline characteristics and procedural outcomes
A total of 100 patients (140 lesions) were enrolled. Baseline characteristics demonstrated a high-risk profile including 34% women, 39% diabetics, and 57% with previous MI (Table 1).
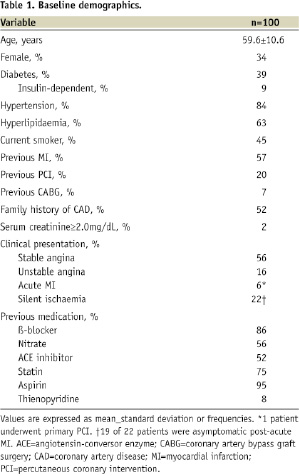
Fifty-six percent of patients presented with stable angina, and 22% presented with ACS (<48 hours) including acute MI in 6%. Table 2 shows the lesion characteristics.
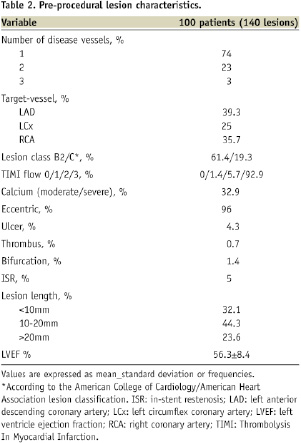
There were 1.4 lesions per patient, 27% had multivessel disease; LAD and RCA were the predominant lesion locations; and 80.7% had complex lesion profile (type B2/C). Overall, 71% of patients had “off-label” indications for PCI.
Procedural outcomes are shown in Table 3.
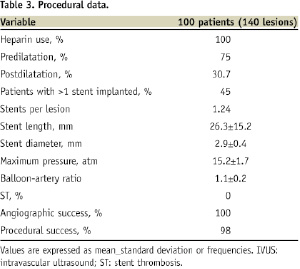
In total, there were 174 ZES implanted (1.24 stents per lesion). All stents were successfully deployed at high pressure (>12 atm). Lesions were commonly predilated, and 80 lesions (57.1%) were treated with a single stent. There were two bifurcation lesions with significant involvement of both branches, which were treated with double-stenting techniques. Total nominal stent length was 26.3±15.2 mm and the stent:lesion length ration was ~1.6. Glycoprotein IIb/IIIa inhibitors were used in only 7% of cases. All lesions achieved angiographic success, and procedural success was 98% (two patients developed postprocedural non-Q MI). There were no cases of ST during hospitalisation. Furthermore, there was neither in-hospital death nor TLR.
Angiographic analysis
Mean lesion length was 16.0±11.3mm and RD was 2.69±0.20 mm. At 6-month angiographic follow-up (95% of patients; 95.7% of lesions), in-stent % DS was 21.5±16.2; in-stent restenosis was 6.7% (9/134 lesions) and in-segment restenosis was 8.2% (11/134 lesions). Conversely, in-stent LLL was 0.66±0.44 mm, and in-segment LLL was 0.37±0.52 mm (Table 4).

Also, the mean length of restenosis was 13.7±6.8 mm.
Independent predictors of in-segment restenosis (10 patients/11 lesions) were evaluated using a multivariate model. Angiographic features did not impact restenosis, and diabetes was the only significant independent predictor (OR=15.27 [95%CI 2.45-95.04], p=0.003). A comparison between patients with versus without diabetics demonstrated in-stent restenosis 12.1% in diabetics (7/58 lesions) versus 2.6% in non-diabetics (2/76 lesions), p=0.03; and in-segment restenosis 15.5% in diabetics (9/58 lesions) versus 2.6% in non-diabetics (2/76 lesions), p=0.009. Among the overall restenotic lesions, a “focal” restenosis pattern was found in 45% (5/11 lesions), and all “diffuse” restenosis (6/11 lesions) including two occlusive restenosis (TIMI=0 in one lesion, and TIMI=1 in the other) were found in stents placed in diabetics.
IVUS outcomes
Serial IVUS at postprocedural and 6-month follow-up with adequate image acquisition and quality for off-line analysis was available in 106 lesions. The mean minimum lumen area (MLA) at postprocedural was 5.88±1.89 mm2 versus 4.35±2.12 mm2 at follow-up, p<0.0001. Acute ISA was found in 4.9% (all at the stent “edges”), and all were resolved at 6-month follow-up. There was no late acquired ISA. Table 5 reports the overall volumetric IVUS results.
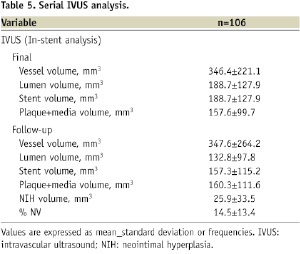
NIH volume was 18.1±35.4 mm2, and %NV was 14.4±13.4%; comparing diabetics versus non-diabetics, there was a non-significant trend towards higher obstruction in diabetics (16.3±14.2% versus 13.1±12.9%, p=0.17); however, follow-up IVUS was not performed in five restenotic lesions in diabetics.
Clinical follow-up
There was no MACE from discharge to 30-day follow-up. At 12-month follow-up (available in all patients), 3.6% of lesions (5/140 lesions) underwent TLR, representing 4% of patients. The majority of TLR were re-PCI (4 PCI versus 1 CABG), and was more frequent amongst diabetics: 7.7% (3/39 patients) versus 1.6% (1/61 patients), p=0.29. The two patients who presented with occlusive restenosis at angiographic follow-up were asymptomatic and had no reports of clinical events or re-hospitalisation during the follow-up period. Overall cumulative MACE at 12-month was 6% (Table 6).
Importantly, there were no cases of ST or death up to 12-month. Figure 1 shows the Kaplan-Meier event free survival curve for MACE up to 12-month follow-up.
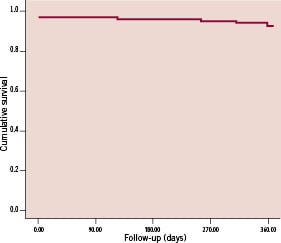
Figure 1. Kaplan-Meier survival curve for MACE up to 12-month follow-up (n=100).
Discussion
Previous trials including patients with selective inclusion criteria demonstrated encouraging results with the ZES, including in-stent LLL of 0.61 mm, 0.61 mm, 0.60 mm, and 0.67 mm, and in-stent restenosis of 5.4%, 9.4%, 9.2%, and 13.3% in the Endeavor I, II, III7-10 and IV* trials, respectively (M. Leon, personal communication, Transcatheter Cardiovascular Therapeutics 2007. Washington, DC, USA). The current study confirmed the results from the previous Endeavor series, including similar in-stent LLL (0.66 mm) and in-stent restenosis of 6.7%. This demonstrates the consistent performance of the ZES and its effectiveness on inhibiting NIH formation across different subsets, including “real-world” patients from our series. In addition, the low rates of MACE found in our study confirmed the remarkable safety profile of the ZES. Importantly, all patients from our analysis remained on thienopyridine therapy for only three months and there were no thrombotic events up to 12 months. Recent reports counting 2,057 patients treated with ZES enrolled in prospective, randomised and non-randomised studies have demonstrated that, to date, the overall ST was 0.5%, including absence of very late (>1 year) ST in the Endeavor I (4-year follow-up), Endeavor II (2-year follow-up), Endeavor II Access Registry (2-year follow-up), and Endeavor III (2-year follow-up) trials.11,12 Such outcomes were not seen with first generation DES.6,19-21
Overall, sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) have demonstrated to significantly alter the patterns of restenosis following stenting, shifting from “diffuse” to “focal” pattern in the majority of cases; however, with uncoated stents, the majority of cases with restenosis (especially more complex subsets) presented with a “diffuse” pattern.1,2 Hence, the mean restenosis length found in our study was higher than expected considering DES. Also, the late lumen loss observed with ZES is considerably higher compared to the late lumen loss observed with SES and PES. These may be related to the relatively faster drug release in ZES compared to SES and PES.1,2,8,22 However, the clinical impact of such findings is still controversial. Previous analysis had suggested that greater neointimal inhibition may be associated with delayed re-endothelisation and vessel enlargement; also, polymeric drug-carrier components used in first-generation DES have been demonstrated to cause an intensive local inflammatory response that may lead to extensive positive remodelling in the treated segment.23-25 Durable polymers are used in the SES, PES and ZES systems; however, the PC polymer used in the ZES mimics a neutral and naturally occurring phospholipid – the main component of cell membranes whose properties include resistance to platelet activation and adherence and absence of inflammatory or hyperplastic responses.14,15,26 Importantly, several recent reports have suggested an association between late acquired ISA and very late (>1 year) stent thrombosis.27-29 Late acquired ISA is a relatively common finding at follow-up with SES and PES (8.0-12.1%).30-32 With ZES, however, no late acquired ISA was found in the current study as well as in the Endeavor I IVUS substudy, and only 0.5% (one patient/lesion) presented with this phenomenon at eight months follow-up in the Endeavor III IVUS substudy.7,10 These findings suggest that the increased late lumen loss observed in ZES with PC coating may be counterbalanced with complete vessel healing and improved long-term clinical safety. Still, larger studies with longer-term clinical follow-up (>12 months) are needed to confirm this hypothesis.
Nevertheless, a study assessing the impact of a novel polymer matrix design to extend drug elution in the Endeavor DES system was recently reported. In this first-in-man trial (Resolute Trial), a durable polymer – BioLinx™ system consisting of a blend of three different components, including hydrophobic C10 and hydrophilic C19 polymers, as well as polyvinyl pyrrolidinone, was used as drug carrier for the Endeavor™ Resolute™ ZES (Medtronic Vascular, Santa Rosa, CA, USA).33 Despite having identical dose of zotarolimus , the drug-elution was slower in the Endeavor Resolute ZES (85% by 60 days, and remaining by 180 days) compared to ZES with PC coating used in our study.8,33 Overall, there were 130 patients included, and angiographic follow-ups at four and nine months demonstrated, respectively, in-stent late lumen loss of 0.12 mm and 0.22 mm, and in-stent binary restenosis of 0% and 1%.33 These results were comparable to the first-in-man trial with SES.34 In addition, in the late follow-up of the Resolute Trial (two years), there was only 1.5% TLR rate and no stent thrombosis (I. Meredith, personal communication, Transcatheter Cardiovascular Therapeutics 2008, Washington, DC, USA).
Furthermore, several studies had demonstrated that late clinical outcomes with first-generation DES in diabetics may be compromised by an excessive risk of re-interventions and thrombosis.35,36 In our analysis, diabetics experienced ~4-fold increase in restenosis, but no significant impact on TLR was observed (p=0.29). By protocol, TLR was indicated only in cases of angiographic restenosis associated with of symptoms of angina (or equivalent) and/or a positive functional test for ischaemia. Of the eight diabetic patients (nine lesions) with restenosis found in our study: three patients (including four lesions) underwent TLR; three patients had angiographic stenosis ~70%, but were asymptomatic with negative functional test for ischaemia, therefore a re-intervention was deferred; and two patients had restenotic lesions with unfavourable morphology for re-interventions, being maintained on optimised medical therapy. At this point, the presence of diabetes comes out as a factor that has somewhat blunted the overall benefits associated with PCI and DES,3,35,36 and efforts to improve outcomes in this high-risk subset are clearly needed. Importantly, despite the overall benefits associated with ZES in our analysis, diabetes remained an independent predictor of angiographic restenosis; therefore, considering the hyperproliferative vascular response often observed in diabetics,37 we may speculate that greater efficacy on inhibiting neointimal proliferation may be preferable while choosing a DES in this subset.
Limitations
This was a prospective, non-randomised study, comprising a relatively small sample of minimally selected patients (exclude lesions located in saphenous vein grafts) from a single clinical institution. However, it was largely represented by high-risk clinical and lesion subgroups and the majority of patients complied with angiographic and IVUS studies (95% and 88% follow-up, respectively). Also, all patients completed 1-year clinical follow-up. Importantly, recent studies have suggested that longer-term angiographic follow-up (>8-month) rather than 6-month may be preferable to accurately account for all restenosis cases; therefore, further studies including angiographic assessment at >6-month follow-up will be necessary to confirm our findings. Finally, IVUS data provides 6-month follow-up results including 0% rate of late acquired ISA; even though such rate is markedly lower than first generation DES over similar follow-up period, longer follow-up is required to confirm the maintenance of this result with ZES.
Conclusions
The ZES demonstrated favourable angiographic and IVUS results at 6-month follow-up in “real-world” patients with complex coronary lesions, including overall in-stent late lumen loss comparable to previous randomised analysis (0.66mm) and absence of late acquired ISA. At 1-year, cumulative MACE rate was 6% (including 4% TLR), and there were no safety concerns including absence of death and ST.

