Abstract
Aims: Mechanical complications contribute to bare metal and first-generation drug-eluting stent (DES) failure. However, the importance of the mechanical complications of second-generation DES remains unclear. We report mechanical complications associated with everolimus-eluting stent (EES) failures.
Methods and results: We retrospectively analysed 177 consecutive EES-treated lesions in 136 patients who underwent intravascular ultrasound (IVUS) at follow-up. Mechanical complications were identified in 17 patients (five stable angina, 10 unstable angina, two non-ST-elevation myocardial infarction [NSTEMI] without angiographic thrombus). Fifteen (88.2%) were treated with repeat revascularisation. By IVUS, there were 16 focal (94.1%) and one diffuse (5.9%) in-stent restenoses. Complete stent fracture with separation was seen in only one, partial stent fracture with separation was seen in three, and in 13 there was longitudinal deformation (n=2) or stent strut fracture (n=11) with overlapping of the proximal and distal stent fragments. In 13 EES with evidence of overlapping in the setting of either fracture or deformation, there was a 35.5±12.2% smaller stent area compared to the adjacent proximal and distal stent fragments, and >50% neointimal hyperplasia in 12 (92.3%).
Conclusions: We found EES mechanical complications, often followed by longitudinal deformation or fracture leading to excessive neointimal hyperplasia, in-stent restenosis, and repeat revascularisation.
Introduction
Everolimus-eluting stents (EES), second-generation drug-eluting stents (DES), have been designed to improve safety and efficacy compared to first-generation DES1-3. EES are characterised by thinner struts (81 µm), a lower amount of drug released through a durable polymer, and a flexible cobalt-chromium alloy metallic scaffolding. Using intravascular ultrasound (IVUS), mechanical complications (beyond chronic underexpansion) which contribute to stent failure have been identified with bare metal stents and first-generation DES4-6; however, they have never been studied in second-generation EES. Compared to coronary angiography, IVUS may provide more detailed and reliable information regarding stent implantation and reasons for failure. Accordingly, the goal of the present study was to assess, using IVUS, mechanical complications after EES implantation which may have contributed to EES restenosis.
Methods
PROTOCOL DESIGN
We retrospectively analysed 177 consecutive EES-treated lesions in 136 patients who underwent IVUS follow-up who had either symptoms or evidence of ischaemia by non-invasive imaging from October 2010 to February 2012 at our institution (New York Presbyterian Hospital, New York, NY, USA). This study was approved by the institutional review board, and written informed consent was obtained from all patients.
ANGIOGRAPHIC ANALYSIS
Qualitative and quantitative angiographic analysis was performed by two independent cardiologists (KY, PG) who were blinded to the clinical and IVUS data. Stent fracture was classified as type I (minor), type II (V-form), type III (complete separation without displacement), and type IV (complete separation with displacement) according to Popma’s classification7. Quantitative coronary analysis was performed with the Cardiovascular Measurement System (Medis, Maastricht, The Netherlands). Minimum lumen diameter (MLD) and mean reference diameter (RD) were used to calculate diameter stenosis (DS=[1-MLD/RD]·100). Late loss was the change in MLD from final percutaneous coronary intervention to follow-up.
IVUS IMAGING ANALYSIS
IVUS imaging was performed after 0.1 to 0.2 mg intracoronary nitroglycerine using a commercially available IVUS system (iLab with 40-MHz Atlantis SR Pro catheters; Boston Scientific, Fremont, CA, USA, or Revolution with 45-MHz catheters; Volcano Therapeutics, Rancho Cordova, CA, USA). Stent fracture was diagnosed by the agreement of two independent cardiologists (SI, AM) who were blinded to clinical and angiographic data.
Quantitative IVUS analysis was performed using computerised planimetry (echoplaque; INDEC Medical Systems, Mountain View, CA, USA). IVUS measurements included the cross-sectional areas of the external elastic membrane, lumen, stent, and intimal hyperplasia (IH=stent minus intra-stent lumen). Focal in-stent restenosis (ISR) was defined as an in-stent minimum lumen area <4 mm2 with significant IH (% IH area >50%) that was ≤10 mm in length; diffuse ISR was defined as an in-stent minimum lumen area <4 mm2 with significant IH that was >10 mm in length8,9.
STATISTICAL ANALYSIS
Statistical analysis was performed using StatView 5.0 (SAS Institute Inc., Cary, NC, USA) and SAS software, version 9.1 (SAS Institute Inc.). Categorical variables were summarised as frequencies. Continuous variables were presented as mean±SD and compared between groups using the Mann-Whitney U test. Intraobserver and interobserver variability for the diagnosis of stent mechanical complications by IVUS were measured by κ test of concordance. A p-value <0.05 was considered statistically significant.
Results
Seventeen EES-treated lesions (9.6%) in 17 patients had IVUS evidence of a mechanical complication. Clinical, lesion, and procedural characteristics at the time of EES implantation are shown in Table 1. The median patient age was 64 years, and 76.5% were men. EES mechanical complications were mainly located in the right coronary artery (70.6%).
The duration between the EES implantation procedure and follow-up was 441±317 days. At follow-up, five patients presented with stable angina, 10 patients presented with unstable angina, and two patients presented with non-ST-segment elevation myocardial infarction (NSTEMI) without angiographic thrombus. Fifteen (88.2%) were treated with repeat revascularisation.
There was no angiographic evidence of longitudinal stent deformation or fracture at the time of EES implantation. At follow-up using the Popma classification of angiographic stent fracture, there were no type I, four type II, two type III, and three type IV fractures; the rest had no evidence of fracture or longitudinal stent deformation. Quantitative angiographic data are shown in Table 2.
IVUS FINDINGS
By IVUS, there were 16 focal (94.1%) and one diffuse (5.9%) in-stent restenoses. Maximum percentage neointimal hyperplasia (NIH) measured 60.3±13.3%.
Complete stent fracture –defined as separation of the stent into ≥2 pieces by image slices with no visible stent struts– was seen in only one patient. Partial stent fracture –defined as the absence of struts over ≥1/3 of the stent circumference with separation of the proximal and distal fragments– was seen in three patients. In 11 lesions, there was a single arc of double layers of stent struts in the same circumference on consecutive frames in the middle of a single EES, suggesting stent fracture followed by longitudinal overlapping. In two lesions, there were at least two separate arcs of stent metal containing multiple layers of stent struts within the same or adjacent image slices, suggesting longitudinal deformation at the proximal stent edge. The maximum angle of overlap without deformation was 103.6±43.4°. Cases with fracture with overlap or longitudinal deformation were associated with a 35.5±12.2% smaller stent area compared to the adjacent proximal and distal stent fragments, NIH that averaged 62.7±9.9% with >50% NIH in 12/13 (92.3%), and the minimum lumen area being co-located at this site in 10 (76.9%). Of 13 EES mechanical complications with overlapping of stent fragments, 10 EES mechanical complications (76.9%) were observed in the proximal edge (within 5 mm from the proximal edge of the stent). On the other hand, there were no EES mechanical complications in the distal edge. Quantitative and qualitative analysis and examples are shown in Table 3 and Figure 1 - Figure 3 (Moving image 1 - Moving image 4).
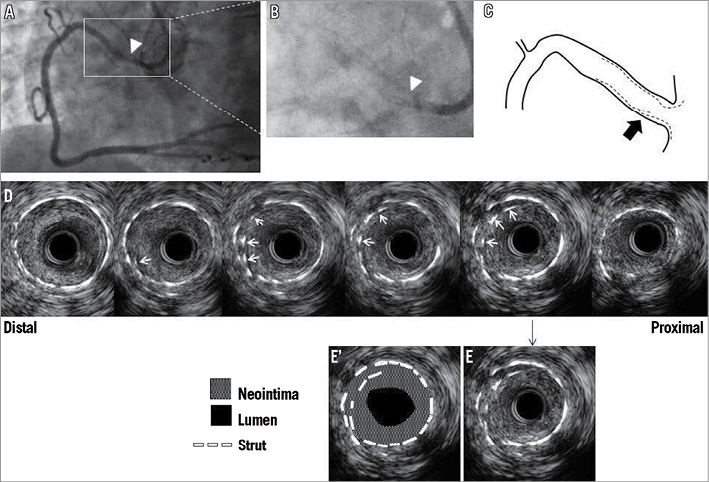
Figure 1. Representative IVUS images of EES fracture at follow-up. A) Angiogram of the RCA shows focal restenosis near the ostium (white arrowhead). B) Magnified angiogram without dye fails to show fracture, white arrowhead corresponds to the fracture site determined by IVUS (D). C) Cartoon - black arrow indicates fracture site. In D) double layered struts (white arrows) appear in the same circumference of consecutive frames indicating fracture and subsequent longitudinal overlapping. E) and E’) show same frame. Neointima fills the space between double layered struts and the space within the inner layers of struts. MLA measures 1.8 mm2 with 70.2% of neointimal hyperplasia. EES: everolimus-eluting stent; IVUS: intravascular ultrasound; MLA: minimum lumen area; RCA: right coronary artery
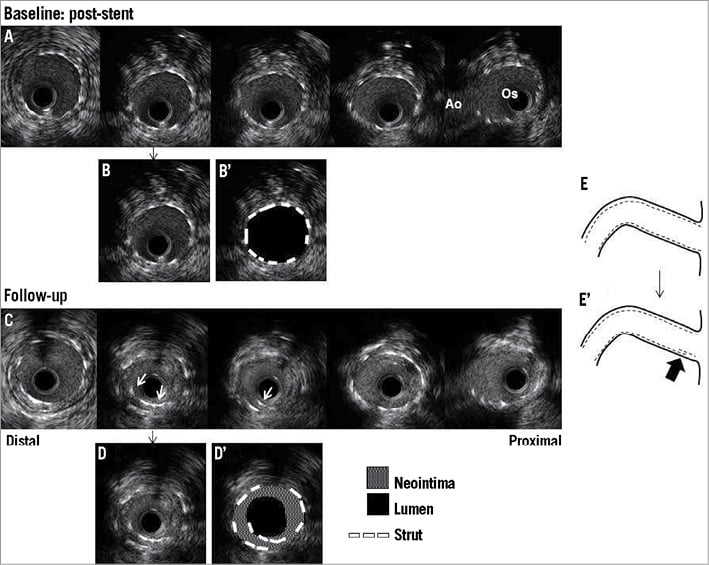
Figure 2. Representative baseline and follow-up IVUS images of EES fracture within the RCA. A) Post-procedural IVUS image at baseline. There was no evidence of deformation or fracture. B) and B’) show same frame. MSA measured 6.9 mm2. C) Corresponding follow-up IVUS images two years after the initial procedure. White arrows indicate double layered struts. D) and D’) show same frame. MLA measured 2.7 mm2 with 58.0% of neointimal hyperplasia. E) (baseline) and E’) (follow-up) Cartoons - the black arrow indicates the fracture site. Ao: aorta; EES: everolimus-eluting stent; IVUS: intravascular ultrasound; MLA: minimum lumen area; MSA: minimum stent area; Os: ostium; RCA: right coronary artery
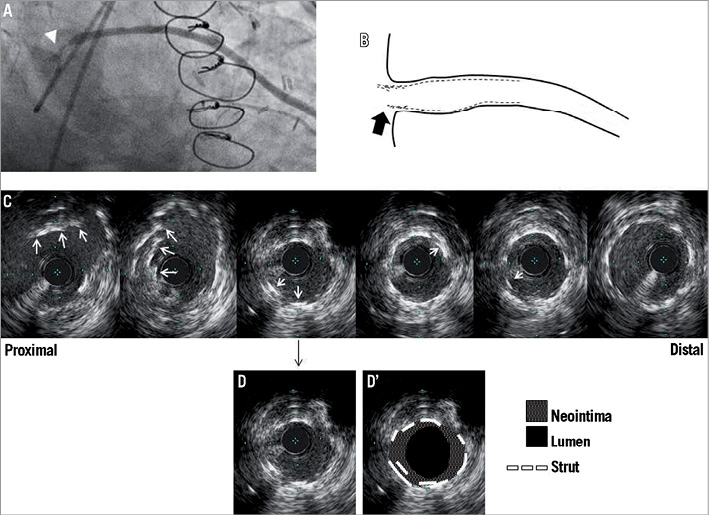
Figure 3. Representative IVUS images of EES deformation at follow-up. A) Angiogram of the saphenous vein graft shows focal restenosis at the ostium (white arrowhead). B) Cartoon - black arrow indicates deformation site. C) Corresponding IVUS images - the white arrows indicate multiple layered struts in the different circumference of consecutive frames. D) and D’) show the same frame. MLA measures 3.2 mm2 with 45.6% of neointimal hyperplasia. EES: everolimus-eluting stent; IVUS: intravascular ultrasound; MLA: minimum lumen area
Comparing the combined group of 13 mechanical complications (longitudinal deformation or partial fracture) that were associated with overlap versus four partial or complete stent fractures that did not have IVUS evidence of overlapping fragments: 1) the minimum stent area at the fracture site (5.3±1.3 mm2 versus 6.8±3.5 mm2) represented 64±12% versus 101±9% of the stent area in the adjacent stent fragments, p=0.0087; and 2) there was more NIH (62.7±9.9% versus 40.2±19.7%, p=0.069). None of these EES mechanical complications was associated with stent-vessel wall malapposition.
We examined interobserver (SJK vs. SI) and intraobserver (SI vs. SI) variability for detection of EES mechanical complications using 40 IVUS images (17 EES mechanical complications, 23 randomly selected controls without mechanical complications). There was good interobserver and intraobserver concordance (k=0.85 [0.54, 1.16] and k=0.90 [0.59, 1.21]).
Discussion
The main findings in this IVUS study of EES mechanical complications were as follows: 1) EES exhibited a unique pattern of longitudinal deformation or fracture with overlapping of the proximal and distal fragments that was associated with a smaller minimum stent area and NIH mainly within the overlap stent segment; 2) EES mechanical complications led to the need for repeat revascularisation in the majority of patients.
MECHANICAL COMPLICATIONS IN THE BARE METAL STENT ERA
Retrospective IVUS analysis of 1,090 ISR lesions in bare metal stents showed that mechanical complications (i.e., stent crush, missing the lesion, and having the stent stripped off the balloon during the implantation procedure) contributed to 49 ISR (4.5%)4. Nevertheless, stent underexpansion more commonly contributed to BMS restenosis compared to these mechanical complications4.
MECHANICAL COMPLICATIONS IN THE FIRST-GENERATION DES ERA
Stent fracture was first recognised in the DES era (Table 4). The reported frequency of first-generation DES fractures ranged from 1% to 2% in clinical studies using angiographic analysis10, and from 0.8% to 8.0% in observational studies of sirolimus-eluting stents (SES) using angiographic and/or IVUS analysis5,6,11-19. Compared to IVUS, coronary angiography had limits in the detection of stent fracture that may have been related to both resolution and the structural pattern of the stent fracture.
The features of first-generation DES fractures have been reported in previous IVUS studies. SES fractures were often observed as complete separation. Although less common than SES fractures, paclitaxel-eluting stent (PES) fractures were often observed as dislocation of stent fragments18,19.
In addition, serial IVUS studies have shown that the main mechanisms of DES restenosis were chronic stent underexpansion (from the time of implantation) and NIH9. Conversely, chronic stent recoil was rare.
MECHANICAL COMPLICATIONS IN THE SECOND-GENERATION DES ERA
The frequency of mechanical complications in second-generation DES has not been studied using IVUS, and even case reports of EES fracture have been rare20,21. The present observational IVUS study showed the incidence of EES-related mechanical complications was 9.6% per lesion. However, angiography detected only 60.0% of cases with IVUS-evident EES mechanical complications, and the sensitivity was lower (45.5%) in the presence of subsequent overlap, whereas all partial or complete fractures without overlap could be detected by angiography. Thus, the frequency of EES mechanical complication, given its unusual structure, having the specific pattern of either deformation or partial fracture with overlap might be underestimated when using angiography alone.
In this current study, we observed a new IVUS pattern that might be specific to EES-longitudinal deformation and/or fracture with overlap of the edges. Because of the unique geometry of the thin (81 µm) flexible cobalt-chromium alloy metallic scaffolding, XIENCE V® (Abbott Vascular, Santa Clara, CA, USA) stents required significantly less force to be elongated than SES22. However, the current study did not identify any specific vascular mechanical forces that led to the development of overlap in the setting of deformation or partial fracture.
In the present study most EES mechanical complications were associated with overlap, and ISR was located at the proximal edge. A recent substudy from RESET revealed that late loss at the proximal edges tended to be greater in the EES group compared to the SES group23.
In the present study, the maximum amount of NIH was located within the overlap segment. In addition, there was evidence of chronic recoil of the edges of the thin flexible cobalt-chromium alloy struts just proximal and distal to the partial fracture or site of deformation: this appeared to contribute to their overlap and, potentially, to the development of ISR. However, it was impossible to exclude chronic stent underexpansion (rather than chronic recoil) since serial IVUS data were not available.
In the present study, two patients with EES mechanical complications presented with NSTEMI, although visible thrombus was not detected by angiography. An IVUS study revealed that stent fracture was identified in 19% of stent thrombosis cases both in BMS and DES24. Moreover, pathological studies have demonstrated a possible association between stent thrombosis and stent fracture25. Recently, Kuramitsu et al demonstrated angiographically that EES fracture was observed in 2.9% and was associated with higher ISR and stent thrombosis. The data might support our current findings26.
Limitations
There were several limitations to the present study. It was retrospective, the frequency of EES mechanical complications was determined from patients undergoing follow-up angiography and IVUS, and the number of EES mechanical complications was small. Therefore, the actual frequency of EES mechanical complications remained unclear. We have only two EES-treated lesions with baseline and follow-up IVUS: both had fracture at follow-up with no IVUS evidence of stent deformation or fracture at baseline. Because in most of the cases we did not have baseline procedural IVUS, we could not eliminate acute mechanical complications, and we could not separate chronic stent recoil from underexpansion at the time of EES implantation. However, our main purpose in the present study was to clarify the mechanism and features of EES mechanical complications by IVUS using consecutive IVUS data in patients treated with EES. Therefore, we think the present study is still meaningful, despite its limitations.
Conclusion
Using IVUS in patients previously treated with EES stents, we observed a unique pattern of EES mechanical complication: longitudinal deformation or fracture with overlap, leading to excessive neointimal hyperplasia, in-stent restenosis, and repeat revascularisation.
Acknowledgement
The authors thank Khady N. Fall, MD, for her assistance in acquiring IVUS data.
| Impact on daily practice The major findings in the present study were: 1) EES exhibited a unique pattern of longitudinal deformation or fracture with overlapped segments, and 2) EES mechanical complications led to excessive neointimal hyperplasia, in-stent restenosis, and repeat revascularisation. In daily practice, the recognition of such mechanical complications will be useful to prevent or explain stent failure. |
Conflict of interest statement
G.S. Mintz: grant support, consultant –Volcano Corporation and Boston Scientific Corporation; K.H. Yun: grants from the Sung San Fellowship at Wonkwang University, Iksan, South Korea; G. Weisz: InfraReDx; J.W. Moses: consultant –Boston Scientific Corporation, Cordis; G.W. Stone: consultant –Boston Scientific, InfraReDx, and Volcano Corporation; A. Maehara: grant support, consultant – Boston Scientific Corporation. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Representative IVUS movie of EES fracture at follow-up.
Moving image 2. Representative IVUS movie of EES fracture at baseline.
Moving image 3. Representative IVUS movie of EES fracture at follow-up.
Moving image 4. Representative IVUS movie of EES deformation at follow-up.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Representative IVUS movie of EES fracture at follow-up.
Moving image 2. Representative IVUS movie of EES fracture at baseline.
Moving image 3. Representative IVUS movie of EES fracture at follow-up.
Moving image 4. Representative IVUS movie of EES deformation at follow-up.

