- coronary angioplasty
- PCB
- DES
- restenosis
Abstract
Aims: After the excellent results of the PACCOCATH ISR and PEPCAD II trials in in-stent restenosis, paclitaxel-coated balloon (PCB) still has to prove its efficacy in native coronary disease.
Methods and results: In the PICCOLETO randomised trial, patients with stable or unstable angina undergoing PCI of small coronary vessels (≤2.75 mm) were randomised to the Dior PCB (28 patients) or the Taxus DES (29 patients). The primary study endpoint was percent diameter stenosis at 6-month angiographic follow-up (non-inferiority); secondary endpoints were angiographic binary restenosis and major adverse cardiac events (MACE: death, Q-wave myocardial infarction, TLR) at 9-month follow-up (non-inferiority). The two groups were not dissimilar for clinical and angiographic data. The study was interrupted after enrolment of two-thirds of the patients due to a clear superiority of one study group. The primary endpoint was not met: the PCB group had higher percent diameter stenosis (43.6% vs. 24.3%, p=0.029), angiographic restenosis (32.1 vs. 10.3%, p=0.043), and higher occurrence of MACE (35.7% vs. 13.8%, p=0.054).
Conclusions: Dior PCB failed to show equivalence to Taxus DES regarding angiographic endpoints during PCI of small coronary arteries. We hypothesise that it concerned the lack of efficacy of the device used (which has since been replaced by its second generation) rather than a class-effect in native coronary vessels.
Introduction
Since the results of studies from the group coordinated by B.Scheller et al became available, there has been great enthusiasm around the new technology of paclitaxel-coated balloons (PCB). The first study involved a PCB constructed with the PACCOCATH technology,1 that outperformed plain balloon angioplasty in in-stent restenosis, allowing for lower late lumen loss (LLL), 0.09±0.49 vs. 0.76±0.86 mm (p=0.003). Shortly afterwards, M. Unverdorben and colleagues published the results of the study Pepcad II2, where a newer PCB (SeQuent Please; B. Braun, Melsungen, Germany) that utilised an evolution of the PACCOCATH technology was compared with the Taxus Libertè drug-eluting stent (DES) (Boston Scientific, Natick, MA, USA) in a bare metal stent (BMS) restenosis population. One hundred and thirty-one patients were enrolled in this study, with a primary endpoint of in-segment LLL at 6-months angiography. The PCB was seen to outperform the DES: LLL rate was respectively 0.17±0.42 mm (median 0.09 mm, interquartile range 0.42 mm) and 0.38±0.61 mm (median 0.24 mm, interquartile range 0.55 mm), p=0.03. Moreover, at 12-months, the rate of MACE was 9% and 22%, respectively (p=0.08), mainly driven by a higher target vessel revascularisation (TVR) in the DES group (15% vs. 6% in the PCB group, p=0.15). A new revolution had begun. It was shortly after this that some researchers decided to test the efficacy and safety of this technology in native coronary artery disease.
While waiting for the results of the PEPCAD III study which had not yet been published, a few months earlier we published the results of the PICCOLETO trial3, a mechanistic study comparing the Dior I generation PCB (Eurocor GmbH, Bonn, Germany) with the Taxus Libertè stent (Boston Scientific, Natick, MA, USA) in small coronary vessels with diameter <2.75 mm.
Methods
This single-centre randomised clinical trial aimed at enrolling 80 patients with complete 9-month follow-up. Patients were eligible if they had a clinical indication to PCI in a small vessel and stable or unstable angina at presentation. Myocardial infarction, unstable haemodynamics and serum creatinine >2 mg/dl were the exclusion criteria.
The primary study endpoint was percent diameter stenosis at 6-month QCA (non-inferiority hypothesised). Secondary endpoints were binary angiographic restenosis (defined as restenosis of >50%) and MACE (death, myocardial infarction, target lesion revascularisation) at clinical follow-up (non-inferiority). In the case of an unsatisfying result in the PCB group (coronary dissection, TIMI flow <3 and residual stenosis >50%), a bare metal stent had to be implanted. Predilatation was mandatory only in the DES group, but if gentle balloon deployment in the PCB group was unsuccessful, predilatation with an uncoated balloon was recommended. PCB inflation time was 45 seconds repeated twice, following the manufacturer’s recommendations.
PCI was performed following standard protocols with unfractionated heparin or bivalirudin. Dual antiplatelet treatment with aspirin and clopidogrel was mandatory for 12 months in the DES group, and one month in the PCB group (three months in case of BMS implantation).
The study protocol contemplated a 6-month angiographic follow-up, and a 9-month clinical follow-up.
Results
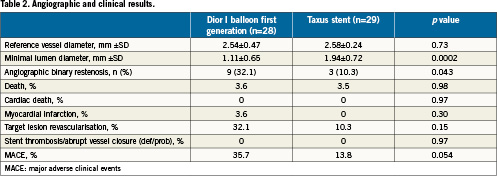
Pre-specified ad interim analysis performed after enrolment and analysis of two-thirds of the patients showed the superiority of one study group, therefore we decided to stop enrolment after having complete data on 28 patients in the PCB group and 29 in the DES group.
Table 1 shows the main characteristics of the patient population. Briefly, baseline and angiographic data showed no significant differences between the two study groups. An exception concerned the number of stents implanted (stents implanted per patient: 0.36 in the PCB group and 1.17 in the DES group, p=0.024) and lesion predilatation (25% and 86.2%, p=0.001). Angiographic and procedural success did not change across study groups.
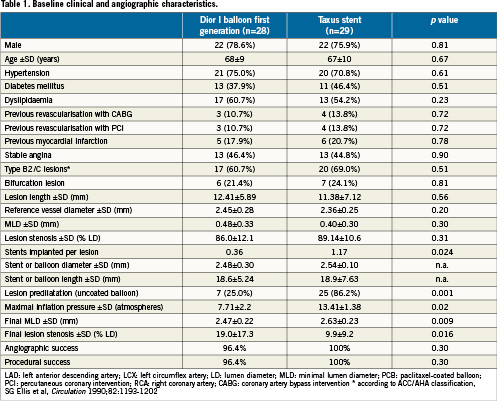
The Taxus group was found superior to the PCB group regarding the primary efficacy endpoint (24.3±25.1% vs. 43.6±27.4%, p=0.029) (Figure 1). The Taxus stent outperformed the Dior balloon also concerning the secondary endpoints such as binary restenosis (10.3% vs. 32.1%, p=0.043) and MACE rates (13.8% vs. 35.7%, p=0.054), the latter mainly due to a higher clinically-driven TLR rate (Table 2). No safety endpoints were raised in this study, as myocardial infarction and death rates were similar between the two study groups.
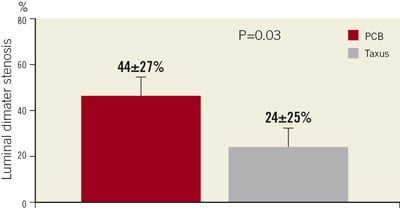
Figure 1. Primary study endpoint. The Taxus group was found superior to the PCB group regarding percent diameter stenosis. PCB: paclitaxel-coated balloon
Discussion
This was the first comparison of a PCB and a DES in a randomised clinical trial of de novo lesions of small coronary vessels, and the PCB failed to demonstrate its equivalence with the Taxus stent.
The analysis of our results astonished us, raising doubts about the overall efficacy of PCB in native vessels. However, due to the good results of previous trials (even in different lesions) and the clear advantages of the PCB technology over a stent strategy (Table 3), these results require further explanation. Particularly, the release kinetics of paclitaxel in PCB differs from that of currently available DES that elute this drug in weeks or months. In comparison, the PCB releases the drug almost entirely in the first 48 hours; however, its biological effect has been discovered to persist for the first 14 days (Figure 2). This is very important, because the process of restenosis is seen in the first days after the baro-trauma induced by angioplasty, and paclitaxel primarily exerts its effect at that time. Moreover, this technology, that allows us to reserve stent implantation only in case of suboptimal immediate results, does not require the implantation of a permanent prosthesis and the use of a polymer, both potentially thrombogenic.
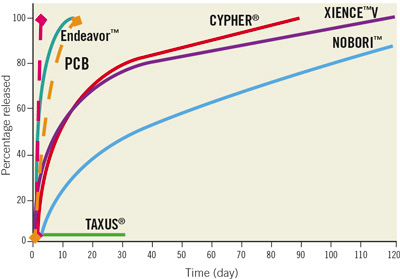
Figure 2. Drug elution time (red dotted line) and duration of biological effect (yellow dotted line) by PCB, as well as main comparators among DES. PCB: paclitaxel-coated balloon
Therefore, it is possible that the true limit of this study is the device used itself, the Dior I generation PCB. It appears probable that this point has also been taken into account by the manufacturer as, in fact, a second generation of the Dior balloon has been recently released.
This second generation Dior balloon still elutes paclitaxel at the same dosage (3mg/mm2) and has been prepared with a similar process including the charging of the balloon with the drug using a micropipetting process with the balloon folded. However, profound differences exist among the two devices. The first generation Dior balloon had a nanoporous surface containing microcristals of pure paclitaxel that are then embedded on the vessel wall at the time of balloon inflation. The second generation Dior II balloon contains shellac as a paclitaxel carrier. Shellac is an inert substance that has already been approved by the FDA as a food additive. It is mostly composed of aleuritic acid, jalaric acid and shelloic acid, and has amolecular weight of about 1000 u. The microporous balloon surface contains a 1:1 mixture of paclitaxel and shellac. Finally, this new technology allows complete paclitaxel deposition with aunique, shorter inflation (around 30 seconds) instead of the two 45-60 seconds inflations previously suggested.
The Dior II generation balloon has been recently tested in an animal model, where it showed a 20-fold higher drug concentration after 45 minutes when compared to its predecessor;4 moreover, tissue paclitaxel resulted in much lower concentration 12 hours after balloon inflation, a result comparable with other PCB in previous studies.
There are several ongoing studies that are testing this new device. Among them, we are about to start the enrolment for the PiccoletoII study, a trial comparing the Taxus Libertè DES with this new generation PCB for the treatment of small coronary vessels.
In conclusion, the PICCOLETO trial failed to demonstrate its equivalence to a DES for the treatment of small vessel disease, both in terms of angiographic and clinical restenosis. Further studies, thanks to newer technological advances, are needed to test the efficacy and safety of PCB in native coronary artery disease. We expect better results, both clinical and angiographic, from the next trial in the native coronary field.
Conflict of interest statement
B. Cortese is a consultant for Eurocor.
References

