Abstract
Aims: To conduct a meta-analysis of studies comparing immediate versus delayed stenting in populations where primary percutaneous coronary intervention (PCI) or early invasive revascularisation was the initial mode of reperfusion.
Methods and results: We identified five non-randomised studies and one randomised trial for a total of 590 patients in studies comparing immediate to delayed stenting in populations where primary PCI or early invasive revascularisation was the initial mode of reperfusion. In non-randomised studies, delayed stenting was associated with a reduction of procedure-related angiographic events (OR=0.13, 95% credible interval [CrI]: 0.03- 0.36). No differences were observed in the rates of major bleeding (OR=0.81, 95% CrI: 0.01-13.42) and major adverse cardiac events (OR=0.40, 95% CrI: 0.09-1.91), between delayed and immediate stenting. In one randomised trial, delayed stenting was associated with a reduction in myocardial infarction during hospitalisation (39% vs. 60%; relative risk [RR]=0.55, 95% confidence interval [CI]: 0.39-0.80). None of the patients assigned to delayed stenting experienced a major adverse cardiac event in the interval between the initial angiogram and the stenting.
Conclusions: Delayed stent implantation is associated with better angiographic outcomes. Randomised trials are required to assess whether delayed stenting translates into better long-term cardiac outcomes.
Introduction
During acute coronary syndromes (ACS), stents are frequently used to stabilise plaques in order to prevent early acute coronary occlusion1,2 and late restenosis3,4. Nonetheless, when implanted in the thrombotic environment typically associated with ACS, stents may provoke coronary emboli and microvascular obstruction5-7. In patients with myocardial infarction, microvascular obstruction post stenting is frequent (30%)8 and has been associated with larger infarct size9,10 and higher mortality rates11.
A mounting body of evidence suggests that delaying stent implantation may be superior to traditional primary stenting in thrombus-laden coronary arteries. In this setting, stent implantation may be deferred until adjunctive anticoagulation and antiplatelet therapy have allowed thrombus burden meltdown12,13.
To date, few studies have explored the potential benefit of withholding stent implantation in acute myocardial infarction when primary or early invasive percutaneous coronary intervention (PCI) strategies were chosen. To our knowledge, there is no prior systematic review of these data. We decided therefore to conduct the present study in order to appraise critically the impact of immediate vs. delayed stenting on procedure-related angiographic events, major bleeding and adverse cardiac events in patients with acute myocardial infarction and patent, yet thrombus-laden coronary arteries.
Methods
In this analysis we primarily sought to assess the quantitative effects of delayed stenting on procedure-related angiographic events in patients with acute myocardial infarction. More specifically, we planned to derive from the analysis an estimate of the odds ratios for procedure-related angiographic events (primary analysis), in-hospital major bleeding, and major adverse cardiac events comparing both revascularisation strategies.
IDENTIFICATION OF STUDIES
Randomised trials are the preferred source of data for meta-analysis. Considering the paucity of randomised trials assessing the strategy of delayed stenting, we elected to include non-randomised studies as well. The report of the methods presented in this paper are compliant with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) consensus statement for non-randomised studies14 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) consensus statement for the randomised controlled trials15.
Studies comparing immediate to delayed stent implantation in patients with STEMI and NSTEMI were considered for the systematic review. Studies were eligible only when the strategy of reperfusion was either primary PCI (in the case of STEMI) or early invasive management (in the case of NSTEMI). The initial coronary angiography had to be performed before the treatment (immediate vs. delayed stenting) was assigned. Therefore, studies comparing early vs. late invasive management following thrombolytics or adjunctive anticoagulation were not considered in this analysis; these treatment strategies have been reviewed elsewhere16-18.
No restrictions were applied regarding language, sample size or length of follow-up. Studies were searched (November 2012) using the MEDLINE, EMBASE, and CENTRAL computerised medical databases. Search strategies included both the Medical Subject Heading term (MeSH) and text word searches without language restriction (Online Table 1A, Table 1B and Table 1C). We searched reference lists of relevant studies manually for additional publications. In addition, trial registers including the World Health Organization (WHO) International Clinical Trial Registry Platform, clinicaltrial.gov, the ISRCTN register, and the metaRegister of Controlled Trials were searched for ongoing or completed studies with potential publication.
Data reported as abstracts, or preliminary reports were not considered. However, relevant abstracts were screened to see whether they were followed by a full report. To this end, we searched the years 2000 to 2011 in the American College of Cardiology, American Heart Association, European Society of Cardiology, Canadian Cardiovascular Conference, Euro-PCR, and the Transcatheter Cardiovascular Therapeutics conference proceedings.
Titles and abstracts of relevant studies were manually reviewed to exclude irrelevant or duplicated publications. The full text of the remaining articles was read entirely. In case of multiple reports from the same study population, the publication with the largest sample size or with the longest follow-up was used. However, multiple reports of a single study were all reviewed for complementary information whenever applicable.
DATA ABSTRACTION AND QUALITY ASSESSMENT
To avoid the possibility that knowledge of the results of the study influenced the perception of the methods, data from the method section of each relevant publication were abstracted without knowledge of the results section. The results sections were thereafter abstracted. At all times, abstracters were blinded to information which had the potential to influence their judgement (authors, titles, journal, institution and country of origin). To reduce bias, data for the methods and for the results section were abstracted on separate forms, by two independent abstractors (XF and EMJ). Discrepancies between abstractors were solved by consensus.
Data were tabulated in standardised data extraction forms. For each selected randomised trial, the general quality of reporting of information was assessed using the validated criteria proposed by Jüni et al19. For each selected non-randomised study, the general quality of reporting of information was initially assessed using the STROBE consensus statement20, which was developed for the reporting of observational epidemiological studies. The specific risk of bias in non-randomised studies was concomitantly assessed using the Newcastle-Ottawa scale for cohort studies21 and the validated checklist proposed by Downs et al22. For every study, an independent quality score was given for the assessment of angiographic events, major bleeding, and long-term cardiovascular events. Data were collected on a customised form adapted from the Cochrane collaboration data collection form for non-randomised studies21. Information included the study design, confounding factors, comparability between groups at baseline, methods used to adjust for confounding and effect estimates (Online Table 2A and Online Table 2B). The quality of studies was not used to adjust their weight in the meta-analysis. Instead quality is reported as an indicator of the validity.
STATISTICAL ANALYSIS
Differences in study methods, patient characteristics, and investigator´s practice patterns mean that the effects of delayed treatment are unlikely to be identical across different studies. We therefore used a Bayesian hierarchical model which accounts for between-study variations in odds ratios7. In this model, the probability (p) of an event within each group of each study varies. To model this between-study variability, the logarithms of the odds ratios of each outcome variable are assumed to have a normal distribution. The mean of the normal distribution of log odds ratios across studies therefore represents the average effect in the studies, and the variance represents the variability between studies. Low-information prior distributions were used throughout, so that the study data drive the final inferences. WinBUGS software, version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK) was used for analyses. Forest plots were produced to display the OR and 95% credible intervals (CrIs) for all major outcomes pooled in our meta-analysis. Credible intervals are the Bayesian analogue to frequentist confidence intervals. To assess publication bias, we generated funnel plots of the effect size (presented as odds ratio) and compared it with the SD for each trial (Online Figure 1).
SENSITIVITY ANALYSES
For the primary analysis, randomised controlled trials and non-randomised studies were analysed as independent data sets and were therefore not regrouped into a single meta-analysis. The context where delayed stenting might have been favoured in observational studies is an important determinant, possibly biasing the association between intervention and outcomes. Recognising this, we decided to perform sensitivity analyses by combining randomised trials and non-randomised studies to explore the case for a future clinical trial and to refine the safety information related to delayed stenting.
Results
SEARCH RESULTS
The initial search retrieved 6,894 references, the vast majority of which reported on non-related topics. As shown in Figure 1, the initial screening retrieved 109 references. A strong proportion of excluded articles compared either: 1) balloon angioplasty vs. stenting; or 2) early vs. late angiogram following successful thrombolytics (in the case of STEMI populations); or 3) on early vs. late invasive management (in the case of NSTEMI populations). Six studies, one randomised trial23 and five non-randomised studies13,24-26 were included in the systematic review, amounting to a total of 590 enrolled subjects, of whom 283 (48%) were treated with delayed stenting. The inter-reviewers agreement on study eligibility was 100%. The quality assessment for the studies included in this systematic review is reported in Online Table 2A and Online Table 2B.
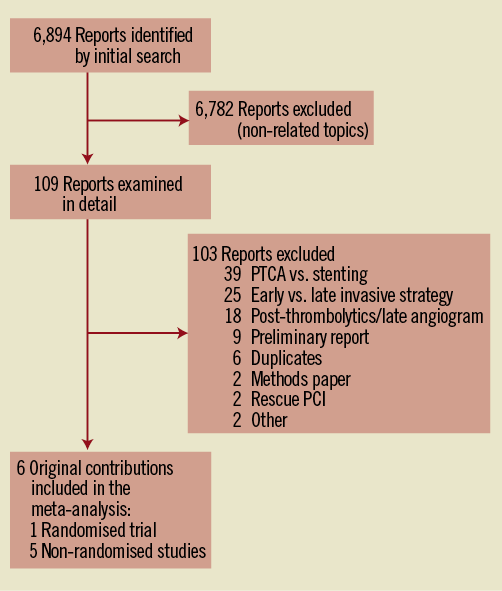
Figure 1. Flow diagram of eligible studies comparing immediate vs. delayed stenting in patients with acute myocardial infarction. NSTEMI: non ST-elevation myocardial infarction; NRS: non-randomised studies; PCI: percutaneous coronary intervention; PTCA: percutaneous transcatheter coronary angioplasty; RCT: randomised controlled trial; STEMI: ST-elevation myocardial infarction
STUDY CHARACTERISTICS
Of the six studies selected, four enrolled patients with STEMI13,25,26 whereas two enrolled patients with NSTEMI23,24. The main characteristics of the selected studies are summarised in Table 1. Most participants were males with an average age varying from 58 to 65 years between studies. Multivessel disease was present in 50% of the patients, with the left anterior descending artery being most frequently reported as the culprit artery. While the rates of aspirin and thienopyridine use were close to 100% in all studies, the rates of GP IIb/IIIa inhibitor use ranged from 39% to 100%.
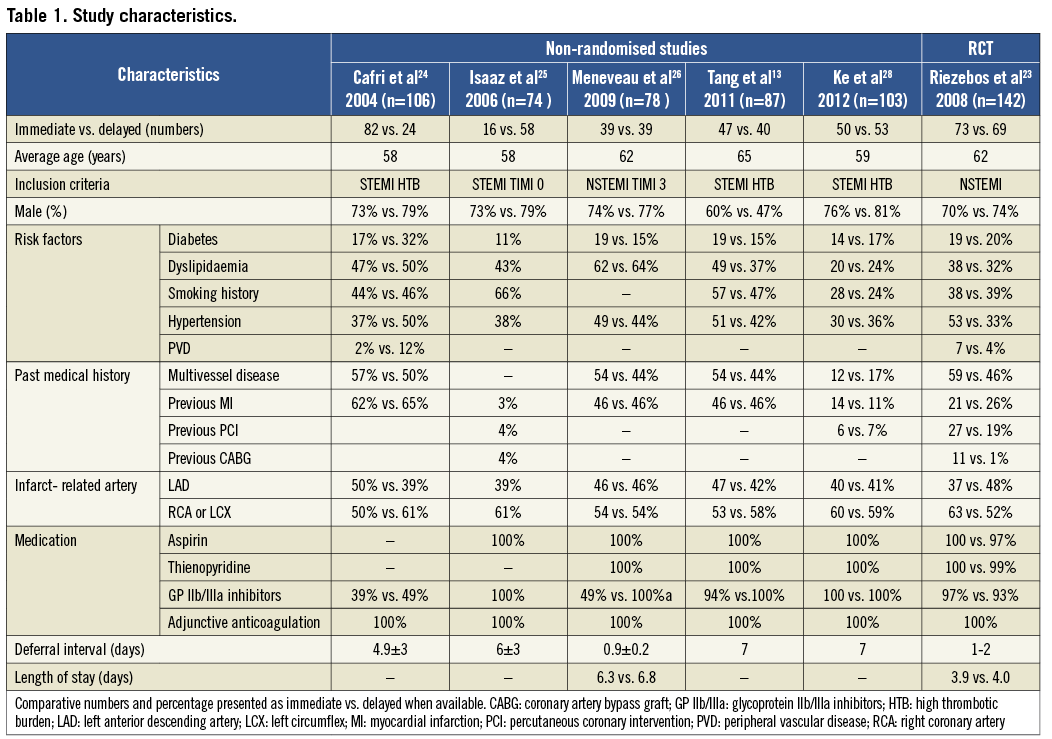
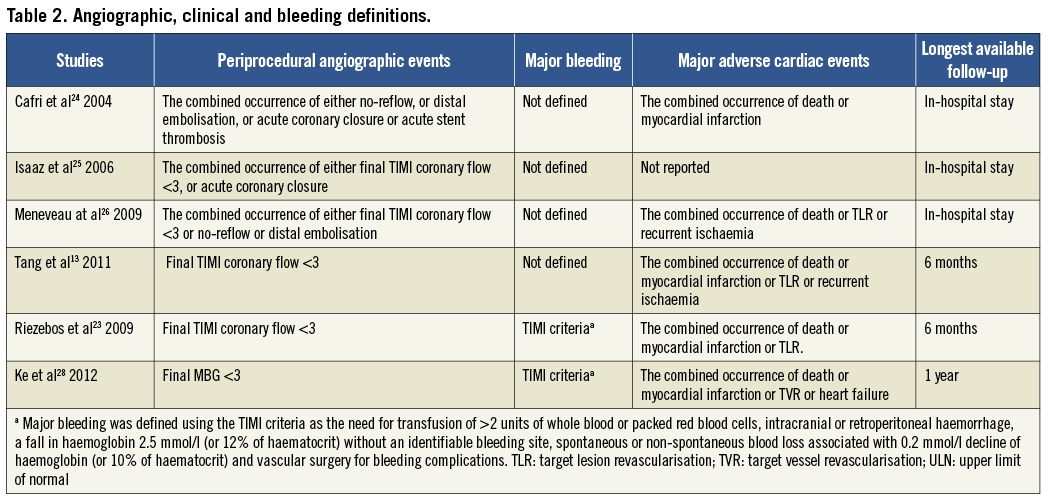
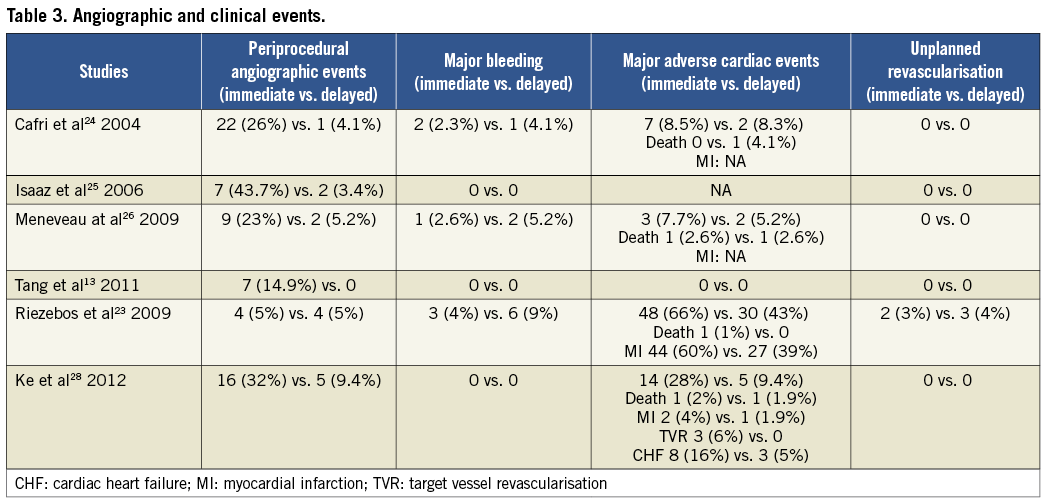
Study-specific definitions and results for procedure-related angiographic outcomes, major bleeding and adverse cardiac events are presented in Table 2 and Table 3, respectively. In all studies, angiographic outcomes were ascertained by the operator using the post-stent angiogram in the infarct-related artery. The criteria for major bleeding were not disclosed, except in two studies where the TIMI criteria were used27,28. In most studies, major adverse cardiac events included death, reinfarction and target vessel revascularisation.
RESULTS OF THE META-ANALYSIS
In the meta-analysis of non-randomised trials, delayed stenting was consistent in showing a clinically substantial reduction in adverse procedure-related angiographic events compared to immediate stenting (OR=0.13, 95% CrI: 0.03-0.36) (Figure 2A). On the sensitivity analysis regrouping randomised and non-randomised studies, the OR for delayed stenting in reducing angiographic events had a similar point estimate, (OR=0.20, 95% CrI: 0.05-063).
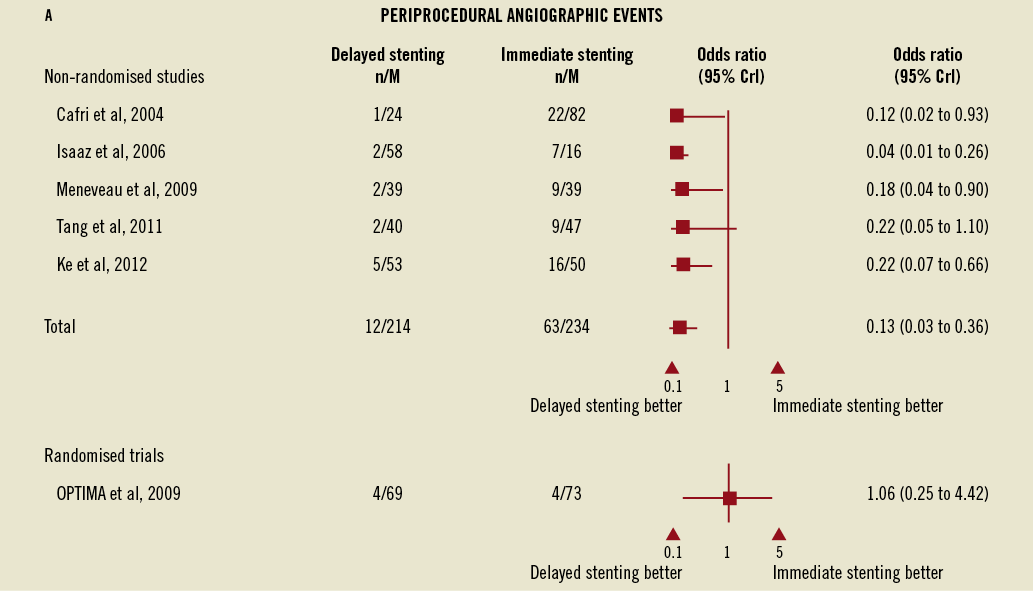
Figure 2. Forest plots for angiographic events, major bleeding, and adverse cardiac events comparing immediate vs. delayed stenting in acute myocardial infarction. A) Forest plots for angiographic events comparing immediate vs. delayed stenting in acute myocardial infarction.
In the non-randomised studies the rates of major bleeding did not vary between immediate and delayed stenting (OR=0.81, 95% CrI: 0.01-13.42) despite the concomitant use of long-term adjunctive anticoagulation, although wide credible intervals preclude definitive conclusions. This observation was consistent with the OPTIMA trial where the differences, although numerically higher, were not significantly different (4.1% for immediate vs. 8.7% for delayed stenting, RR=1.40, 95% CI: 0.86-2.31, Figure 2B), as well as in the sensitivity analysis of all studies combined (OR=2.17, 95% CrI: 0.08-142.85).
The rates of major adverse cardiac events did not differ between delayed and immediate stenting strategies in either the main meta-analysis (Figure 2C) or the sensitivity analysis although, once again, wide credible intervals preclude definitive conclusions.
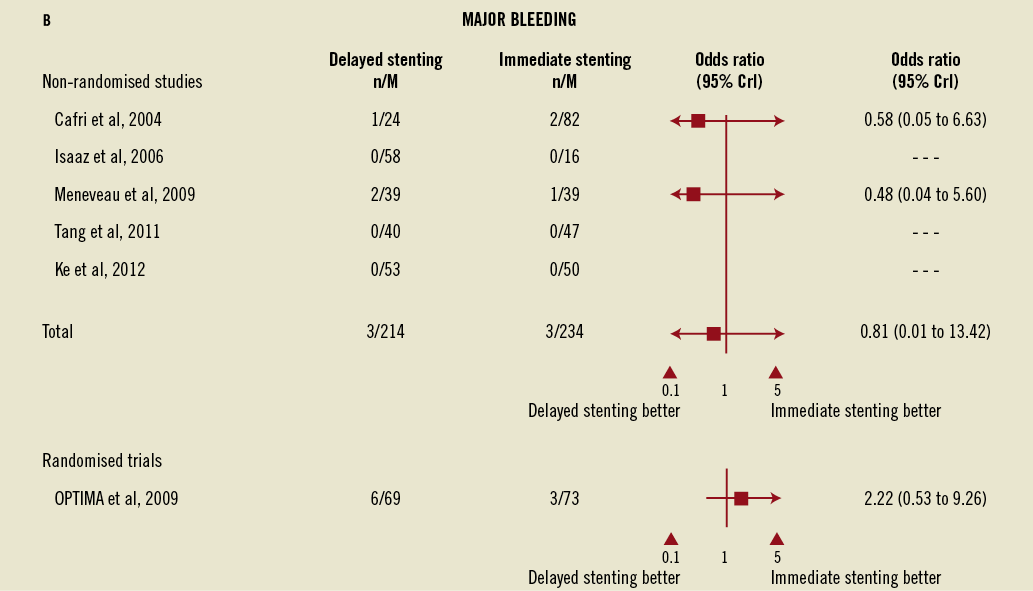
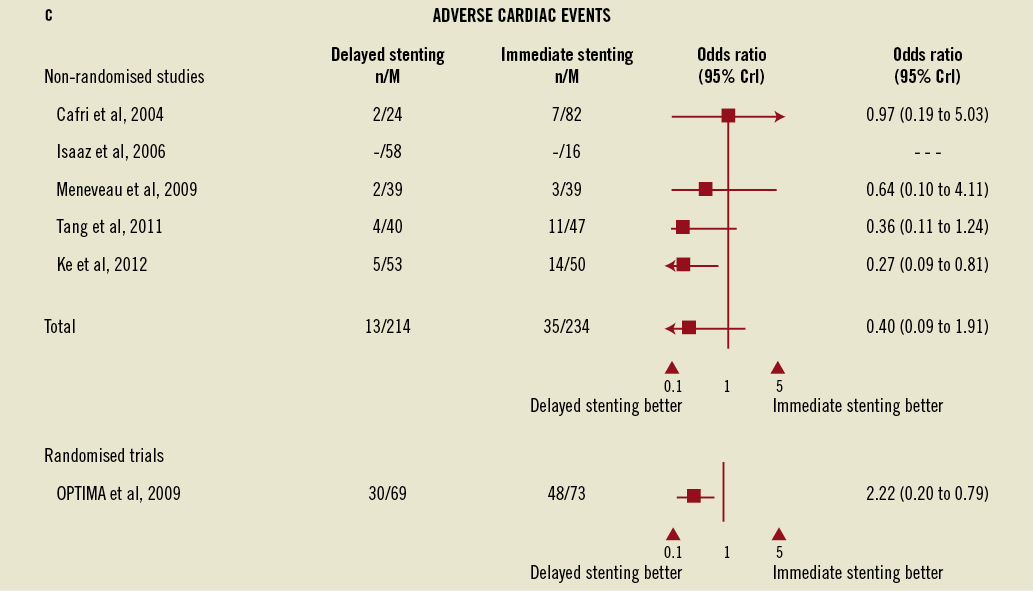
Figure 2. Forest plots for angiographic events, major bleeding, and adverse cardiac events comparing immediate vs. delayed stenting in acute myocardial infarction. B) Forest plots for major bleeding comparing immediate vs. delayed stenting in acute myocardial infarction. C) Forest plots for adverse cardiac events comparing immediate vs. delayed stenting in acute myocardial infarction. CrI: credible interval
In the OPTIMA randomised trial however, delayed stenting was substantially better than immediate stenting in reducing the combined occurrence of death and myocardial infarction and revascularisation at six months (43% vs. 66%; RR=0.66, 95 % CI: 0.48-0.91). This difference was mostly driven by a reduction in the rates of myocardial infarction in the delayed stenting group (39% vs. 60%; RR=0.55, 95% CI: 0.39-0.80) defined as any rise in CK-MB exceeding the upper limit of normal more than once. On the sensitivity analysis combining all studies, a positive association of borderline significance was found between a delayed stenting and a reduction of cardiovascular events (OR=0.4, 95% CrI: 0.18-1.00).
In terms of safety, none of the 283 subjects assigned to delayed stenting experienced an acute coronary reocclusion in the interval between the initial reperfusion and stent implantation (average time of 3.7 days). In one trial, two patients assigned to delayed stenting experienced rest angina which did not lead to a major adverse cardiac event13.
Discussion
The present systematic review assessed the impact of immediate versus delayed stenting in acute myocardial infarction. In patients admitted for STEMI and NSTEMI, delayed stenting (up to seven days) is associated with a significant reduction in procedure-related angiographic events and possibly with a reduction in adverse cardiovascular events. In addition, delayed stenting appears to be safe and specifically not associated with acute ischaemic events in the interval between initial angiography and postponed stenting.
Delayed stenting in thrombus-laden coronary arteries has been proposed as a strategy for preventing distal embolism and no-reflow in acute coronary syndromes13. Some studies have explored the benefits of delayed PCI with intensive antithrombotic agents after fibrinolysis showing angiographic benefits probably linked to the prolonged effect of the antithrombotic treatment before stenting29,30. The present analysis explores a different population of patients characterised by an early angiographic assessment of their infarct-related artery. This early assessment allows a proper evaluation of the coronary anatomy and permits the performance of intermediate reperfusion strategies, such as undersized balloon angioplasty or thrombectomy, to obtain an optimal TIMI 3 flow before delaying stent implantation.
Improved angiographic outcomes provided by delayed stenting, such as the higher percentage of post-PCI TIMI 3 flow, have been associated with a reduction in death, myocardial infarction and repeat revascularisation after PCI31-33. A potential mechanical explanation for the improvement associated with delayed stenting may actually be the reduction in thrombus burden. In all the studies where quantitative coronary analysis was performed13,24,26 a significant reduction in thrombus burden was observed before and after the interval required for delayed stenting. The OPTIMA randomised trial was conducted in patients with NSTEMI and reported a significant reduction in both in-hospital and six-month myocardial infarction although no differences in angiographic outcomes were seen. However, it is not clear if angiographic outcomes were properly assessed since quantitative coronary analysis was not performed, and the high rates of post-PCI myocardial infarction (60% in immediate vs. 39% in deferred stenting) contrasted with the low percentages of post-PCI TIMI <3 flow observed (5% in immediate vs. 6% in deferred stenting). The high incidence of post-PCI myocardial infarction in the OPTIMA trial was however one of the most controversial points as a result of the difficulty to distinguish between procedure-related and on-going myocardial infarction in the immediate PCI strategy. In any case, the inclusion of a comprehensive analysis of the thrombotic burden, quantitative coronary analysis and coronary flow should be included in the design of future randomised trials.
One of the most reassuring findings was the absence of acute coronary reocclusion in the interval between the initial reperfusion and the actual stenting. Of note was the pointer towards a reduction in clinical adverse events and the absence of significant differences in major bleeding rates among groups although the wide credible intervals precluded any definite conclusion. Bleeding events and transfusions have been shown to be independent predictors of mortality in patients with acute coronary syndromes and in patients undergoing PCI34,35. Although the origin of major bleeding is multifactorial, access-related vascular complications account for an important percentage of bleeding and they are also associated with a worse prognosis after PCI36. Unfortunately, we were not able to report the vascular access-site information as data were not consistently provided.
Beyond reduced odds of distal emboli and microvascular obstruction, many other potential advantages favouring delayed stenting could be cited: 1) acute thrombotic plaques, especially in primary PCI, are associated with a 34-40% rate of acute stent malapposition as shown in the IVUS analyses38. Reducing thrombotic burden before stenting could not only reduce embolic events but also allow for a better stent selection which might translate into shorter lengths and larger diameters. 2) Patients with multivessel disease where surgical revascularisation could be an option could have their stenting deferred and reassessed by a multidisciplinary team. 3) Medical compliance could be carefully assessed and allow a better stent selection (bare metal vs. drug-eluting stents). 4) In about 10% of patients, the infarct-related coronary lesions might be treated medically without requiring a stent at all26. 5) In STEMI cases, the repeated angiogram may allow treatment of the non-culprit artery in patients with multivessel disease39. 6) Delayed stenting allows for a proper statin loading before the stenting which has been associated with a reduction in the composite of death, myocardial infarction, unstable angina and repeat revascularisation after PCI40.
Delayed stenting might potentially prolong the stay of a considerable proportion of patients, especially in cases of NSTEMI where patients are often discharged the day following stent implantation. A second intervention might also increase not only the costs but also the risks of a procedure-related complication. Additionally, in the era of the new-generation DES in which the risk of stent thrombosis might be even lower than BMS41, delaying PCI for stent selection (DES vs. BMS) may be less beneficial. Whether the potential reduction in long-term adverse events associated with delayed stenting will offset the costs and potential risks associated with a repeat angiography and a prolonged hospital stay will have to be formally assessed in a cost-effective analysis in a randomised trial. Nevertheless, the strategy may be efficient in patients with STEMI.
LIMITATIONS AND FUTURE DIRECTIONS
Our meta-analysis has obvious limitations. First, the studies included in the analysis were relatively inhomogeneous with regard to the type of disease treated (STEMI vs. NSTEMI) which might have biased the interpretation of the study as a result of the potential differences in the amount of myocardium at risk, thrombotic mechanism, difference in pre-infarction angina or presence of multivessel disease. Second, the deferral interval varied considerably between studies (from 24 hours to one week) so that it is not possible to draw a conclusion about the optimal interval between the index angiogram and stent implantation. Third, the absence of long-term data in the majority of studies does not allow a meaningful conclusion as to the benefit of delayed stenting on hard clinical events such as congestive heart failure or death. The overall number of available patient data limits a differential assessment of efficacy between STEMI and NSTEMI populations. The information enclosed in the manuscript can nonetheless be considered to help design future randomised trials. Fourth, we did not have access to patient-level data so that it was not possible to perform multivariable adjusted odds ratios which would have minimised the risk of inaccurate inference. Finally, we used a random effects approach wherein the final credible intervals depend on the sum of between and within study variances. However, the small number of studies meant that between study variance could not be well estimated, as it is impossible to estimate accurately a variance parameter with only five or six data points (studies). We therefore used a Bayesian approach which accounts for all uncertainty inherent in the problem, including the uncertainty in estimated variance, rather than using a point estimate. This conservative approach results in wider credible intervals than if another technique had been used which ignores uncertainty in the variance parameter.
Whether delayed stenting is better than immediate stenting remains controversial, but this important research question should soon be answered. No less than four registered trials are proposing to compare immediate to delayed stenting in acute coronary syndrome populations: the PRIMACY trial (NCT01542385), the MIMI-trial (NCT01360242), the DANAMI-3 trial (NCT01435408), and the OPTIMASTRATEGY (NCT01462188).
In conclusion, the current study shows that delaying stent implantation in a thrombus-laden coronary artery is associated with better early angiographic outcomes. These results support conducting a larger dedicated trial to assess whether delayed stenting translates into differences in major bleeding rates and better long-term cardiac outcomes, especially among patients with STEMI.
Funding
M. Jolicoeur is supported by a grant from les Fonds la recherche en santé du Quebec (FRSQ), and by la Fondation de l’Institut de Cardiologie de Montréal.
Conflict of interest statement
The authors have no conflicts of interest to declare.
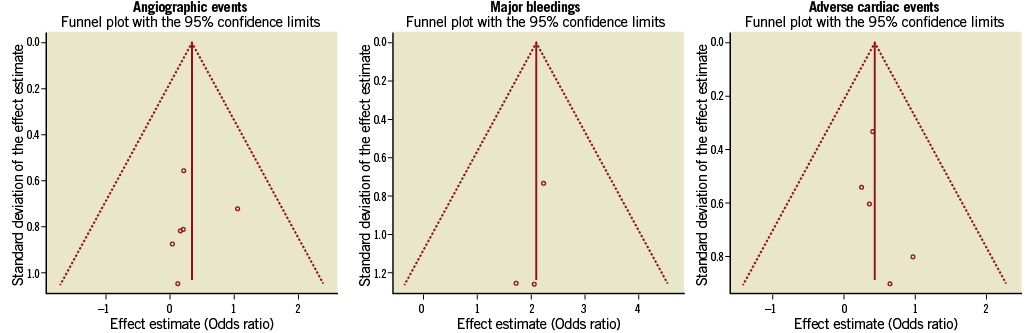
Online Figure 1. Assessment of publication bias. Funnel plots for angiographic events, major bleeding and adverse cardiac events presenting effect estimate vs. standard deviation of effect estimate and 95% confidence interval showing the publication bias and the consistency of the study results around the mean outcomes.

