
Abstract
Aims: To compare an early to a delayed invasive strategy in high-risk patients with NSTE-ACS.
Methods and results: In this prospective multicentre trial, 542 patients hospitalised with NSTE-ACS were randomised to either an immediate (angiography and revascularisation if appropriate <12 hr) or a delayed invasive strategy (>48 hr after randomisation). Patients were eligible if they had two of the following three high-risk characteristics: evidence of extensive myocardial ischaemia on ECG, elevated biomarkers for myocardial necrosis (TropT >0.10 μg/L), and an age above 65 years. Primary endpoint of the study was the combined incidence of death, reinfarction and/or recurrent ischaemia at 30-day follow-up. Secondary endpoints were enzymatic infarct size as assessed by a single cardiac troponin T, at 72-96 hours after admission or at discharge, and the percentage of patients without a rise in CKMB during admission. Median age was 71.9 (interquartile range [IQR] 64.5-78.4) years. Median time between randomisation and start of angiography was 2.6 (IQR 1.2-6.2) hours in the immediate and 54.9 (44.2-74.5) hours in the delayed intervention group. The composite of death, reinfarction and/or recurrent ischaemia at 30 days occurred in 12% of patients and was not significantly different between the two groups (9.9% and 14.2%, respectively, p=0.135). All secondary endpoints and bleeding complications were comparable. Hospital duration was two days shorter in the immediate intervention group (4 days [IQR 2-10] vs. 6 days [IQR 4-12]).
Conclusions: Although no definitive conclusion can be drawn due to a lower than expected prevalence of the primary endpoint, an immediate invasive strategy was safe and feasible but not superior to a delayed invasive strategy in terms of the combined primary endpoint of death, reinfarction and/or recurrent ischaemia at 30 days. These results are consistent with previous randomised trials which studied the effect of timing of angiography in patients with NSTE-ACS. Trial Registration: ISRCTN Register 9230163.
Abbreviations
BPM: beats per minute
GRACE: Global Registry of Acute Coronary Events
IQR: interquartile range
ISRCTN: International Standard Randomised Controlled Trial Number
MI: myocardial infarction
NSTE-ACS: non-ST-segment elevation acute coronary syndrome
NSTEMI: non-ST-segment elevation myocardial infarction
RCT: randomised clinical trial
ULN: upper limit of normal
Introduction
Current guidelines recommend a routinely invasive strategy (angiography and revascularisation if applicable) in patients presenting with non-ST-elevation acute coronary syndrome (NSTE-ACS)1,2. Several randomised clinical trials (RCTs) and meta-analyses have shown that this strategy, compared to a selective invasive treatment strategy, reduces the incidence of cardiovascular death and myocardial infarction (MI) in the medium to long term3-10. This is especially true in patients at higher risk. Troponin elevation and ST depression at baseline appear to be among the most powerful individual predictors of benefit from a routine invasive treatment.
With the exception of indication for emergency angiography and revascularisation, controversy remains about the optimal timing of angiography for patients presenting with NSTE-ACS who are planned to undergo invasive treatment. Some RCTs have shown that early angiography and revascularisation improve clinical outcome11,12, while others showed an increase in infarct size13. Three other trials showed no significant differences in cardiovascular outcome14-16. In the largest of these trials14, a pre-specified subgroup analysis showed a significant reduction in the combined endpoint for death, MI and stroke at six months for patients at highest risk (GRACE score >140).
A meta-analysis of five RCTs17,18 and a recently published updated meta-analysis of RCTs and observational studies19 showed that little or no benefit was found with early intervention. A non-significant decrease in rate of mortality and major bleeding and a non-significant increase in MI was found besides a statistically significant decrease in refractory ischaemia. These findings were limited by heterogeneity in timing of intervention and patient risk profiles.
Although the majority of patients with NSTE-ACS are older and the prevalence of ACS-related complications increases with age, elderly patients are underrepresented in studies investigating the efficacy and hazards of pharmacological and invasive management of NSTE-ACS. Therefore, it is recommended that trials should enrol elderly subjects proportional to their prevalence among the treated population20.
The aim of this study was to compare an early to a delayed invasive strategy in a population of high-risk patients with NSTE-ACS. By defining age above 65 years as one of the high-risk criteria, inclusion of elderly patients was encouraged.
Methods
The objective of the ELISA-3 study, an investigator initiated, randomised, open, multicentre study, was to test the hypothesis that immediate angiography and revascularisation are superior to delayed angiography no earlier than 48 hours after admission, in high-risk non-ST-elevation acute coronary syndrome patients. The trial was conducted in six Dutch hospitals of which one had 24-hour facilities for (primary) PCI and CABG. The study complied with the Declaration of Helsinki, was approved by the ethics committee of the Isala klinieken, Zwolle, The Netherlands, and all patients gave written informed consent before study entry. The study was registered in the ISRCTN Register (ISRCTN39230163).
PATIENTS
Patients were eligible if they were hospitalised with ischaemic chest pain or dyspnoea at rest, with the last episode occurring 24 hours or less before randomisation, and had at least two out of three of the following high-risk characteristics: (1) evidence of extensive myocardial ischaemia on ECG (shown by new cumulative ST depression >5 mm or temporary ST-segment elevation in two contiguous leads <30 min), (2) elevated biomarkers (troponin T >0.10 µg/l or myoglobin >150 µg/l) or elevated CKMB fraction (>6% of total CK), (3) age above 65 years. Exclusion criteria were persistent ST-segment elevation, symptoms of ongoing myocardial ischaemia despite optimal medical therapy, contraindication for diagnostic angiography, active bleeding, cardiogenic shock, acute posterior infarction and life expectancy less than one year.
RANDOMISATION AND TREATMENT
After giving written informed consent, patients were randomly assigned to an immediate or delayed treatment strategy by a web-based computerised system, stratified to the symptoms of patients: chest pain or dyspnoea. In patients assigned to the immediate treatment strategy, angiography was performed as soon as possible but within 12 hours of randomisation. Patients assigned to the delayed treatment strategy underwent angiography no sooner than 48 hours after randomisation, unless clinical instability or recurrent ischaemia despite optimal medical therapy warranted emergency angiography. In patients recruited at a non-PCI centre, the patient was urgently transferred to the interventional centre for angiography and revascularisation in case of randomisation to the immediate intervention group. In case of assignment to delayed intervention, angiography was performed at the local non-PCI centre. Angioplasty was performed according to the local standards of the intervention centre. All patients were treated according to the guidelines. Concomitant medication included a loading dose of aspirin (500 mg orally or I.V.), clopidogrel (600 mg orally) and 5000 IU unfractionated heparin I.V. as soon as possible after diagnosis. In case of angioplasty, Aggrastat (bolus of 25 mg/kg followed by continuous infusion of 0.15/min/kg) was given in both groups, starting shortly before the intervention, for 12 hours. Nitrates, beta-blockers and calcium channel blockers were given at the discretion of the investigator.
ENDPOINTS
The primary endpoint of the study was the combined incidence of death, reinfarction and/or recurrent ischaemia at 30-day follow-up. Death was defined as death from any cause. Recurrent ischaemia was defined as recurrent chest pain associated with new or recurrent ECG abnormalities requiring urgent or repeat angiography or repeat hospitalisation. The definition of reinfarction was related to the presence of elevated CKMB on admission: early reinfarction in patients presenting with a CKMB >upper limit of normal (ULN) was defined as a decrease in CKMB of at least 50% of ULN from a prior peak level to a valley followed by a new increase with a value above the sum of the preceding valley and three times the ULN or the development of new Q-waves in two or more contiguous leads (defined as Q-waves greater than or equal to 0.04 sec in duration or having a depth greater than or equal to one fourth of the corresponding R-wave amplitude). Early reinfarction in patients presenting with CKMB not exceeding the upper limit of normal was defined as a peak CKMB greater than three times the ULN with the exception of cases where the CKMB release curve was unequivocally related to the chest pain episode before randomisation and not to the chest pain episodes after randomisation or development of new Q-waves in two or more contiguous leads. Late reinfarction in patients whose CKMB had returned to (or had remained) normal was defined as peak CKMB greater than three times the ULN or the development of new Q-waves in two or more contiguous leads. MI in patients who underwent CABG was defined as the development of new Q-waves in two or more contiguous leads.
Secondary endpoints were enzymatic infarct size as assessed by a single cardiac troponin T, at 72-96 hours after admission or at discharge, and the percentage of patients without a rise in CKMB during admission. In addition, bleeding complications were assessed. Major bleeding was defined as bleeding with Hb drop of ≥2 mmol/l or a blood transfusion of two or more units.
Pre-specified subgroups included heart rate on admission (under or above 100 beats per minute), pro-BNP, troponin T values (both below versus above median value), cumulative ST-segment depression on admission (<5 mm versus ≥5 mm), patients presenting with dyspnoea versus chest pain, patients with diabetes yes or no, and gender. Post hoc defined subgroups were age, GRACE risk score (both below versus above median value), ST deviation (cumulative ST depression <5 mm versus ≥5 mm or temporary ST elevation) and PCI vs. non-PCI centres.
STATISTICAL ANALYSIS
Data were analysed according to the intention-to-treat analysis. Continuous variables were expressed as median and interquartile range (IQR) and were compared between the intervention groups using a Mann-Whitney U test. Categorical data were described by proportions and compared with the chi-square or Fisher’s exact test. Logistic regression was used to calculate the p-value of the interaction between the effect of the intervention and the pre-specified subgroups upon the primary endpoint. All tests were two-sided and an alpha of 5% was used. Statistical analysis was performed with SPSS (version 20); SPSS Inc., Chicago, IL, USA
SAMPLE SIZE
The sample size of the study was calculated to demonstrate the superiority of immediate angiography compared to delayed angiography. The primary endpoint was the incidence of death, reinfarction or recurrent ischaemia within 30 days of admission. The 30-day incidence of the combined endpoint in the delayed angiography group in this high-risk study population was estimated to be 25% (5% mortality, 8% MI, 12% recurrent ischaemia), based upon previous data in high-risk patients21. An absolute risk reduction of 10% was expected in the patients treated with the immediate catheterisation approach (4% mortality, 4% MI, 7% recurrent ischaemia). At an alpha level of 5% and a beta level of 20%, the number of patients required was 540 (270 per group). An interim analysis was performed according to the probability stopping rules of Snapinn22 after inclusion of 380 patients.
Results
PATIENT AND PROCEDURE CHARACTERISTICS
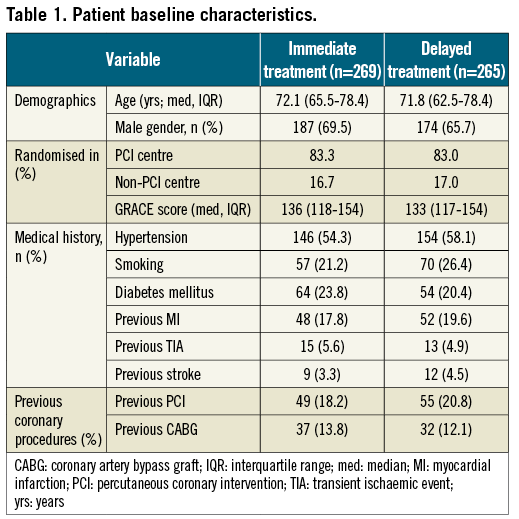
The trial flow chart is shown in Figure 1. Between July 2007 and June 2012, 542 patients were randomised in six hospitals. Ninety-two patients (17%) were randomised at a non-PCI centre. Eight patients were excluded, five because of withdrawn consent and three for major protocol violation. Two hundred and sixty-nine patients were allocated to the immediate intervention and 265 to the delayed intervention group. Baseline characteristics were well balanced between the treatment groups (Table 1). Median age was 72 years: 74% of patients were older than 65 years and the median GRACE risk score was 135.0 (IQR 117-154). Thirty-day follow-up was obtained in 97.9% of the patients.
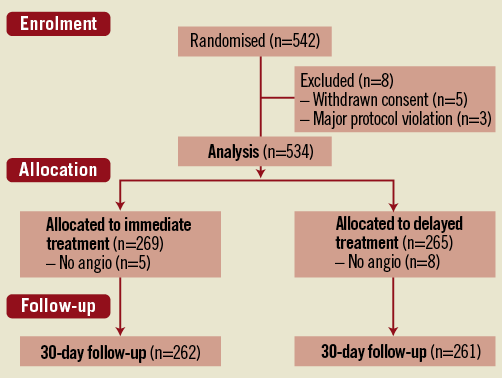
Figure 1. Consort trial flow chart.
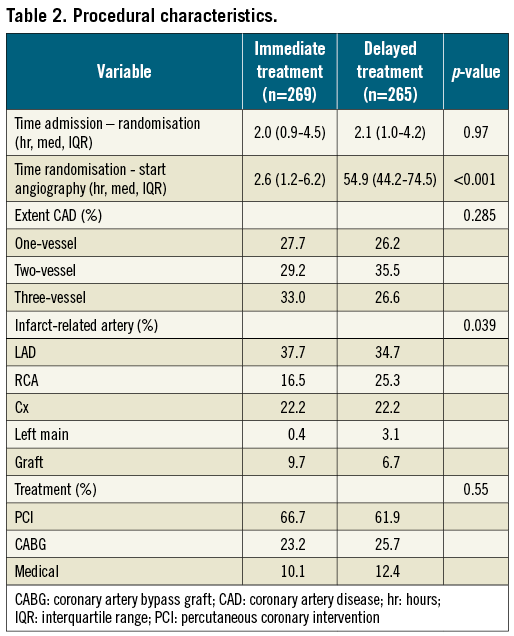
Angiography was performed in 98.1% of the patients in the immediate treatment group and in 97.0% in the delayed treatment group, with median time from randomisation to angiography 2.6 (IQR 1.2-6.2) hours and 54.9 (IQR 44.2-74.5) hours, respectively. Of the patients assigned to immediate intervention, 39 (14%) underwent angiography more than 12 hours after randomisation (median 16.7 hr, IQR 13.6-19.4) because of logistic reasons. Of the patients randomised to delayed intervention, 18 (6.8%) underwent angiography within 48 hours of randomisation due to recurrent ischaemia, five (1.9%) for other medical reasons and 69 patients (26%) for logistic reasons (median time to angiogram 42.7 hr, IQR 31.4-46.2 hr). One, two and three-vessel disease were equally distributed between the two groups, as was the rate of PCI, successful PCI and CABG (Table 2). The use of drug-eluting stents did not differ between the immediate and the delayed treatment group (54.5% and 55.6%, respectively, of patients who received a stent at PCI, p=0.86).
STUDY ENDPOINTS
The primary endpoint of the study, the combined incidence of death, reinfarction and/or recurrent ischaemia at 30-day follow-up, occurred in 9.9% of the patients in the immediate treatment group and 14.2% of the patients in the delayed treatment group (p=0.135) (Table 3). There was no statistically significant difference between the groups in the individual components of the primary endpoint, the rates of death (both groups 1.1%, p>0.99), (recurrent) myocardial infarction (1.9 vs. 0.8%, p=0.45) and recurrent ischaemia (7.6 vs. 12.6%, p=0.058). Analysis of pre-specified and post hoc defined subgroups showed no significant interaction with immediate or delayed treatment upon the primary endpoint (Figure 2 and Figure 3).
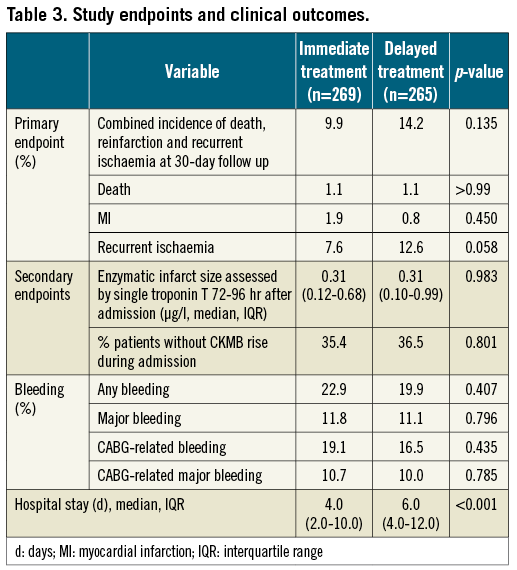
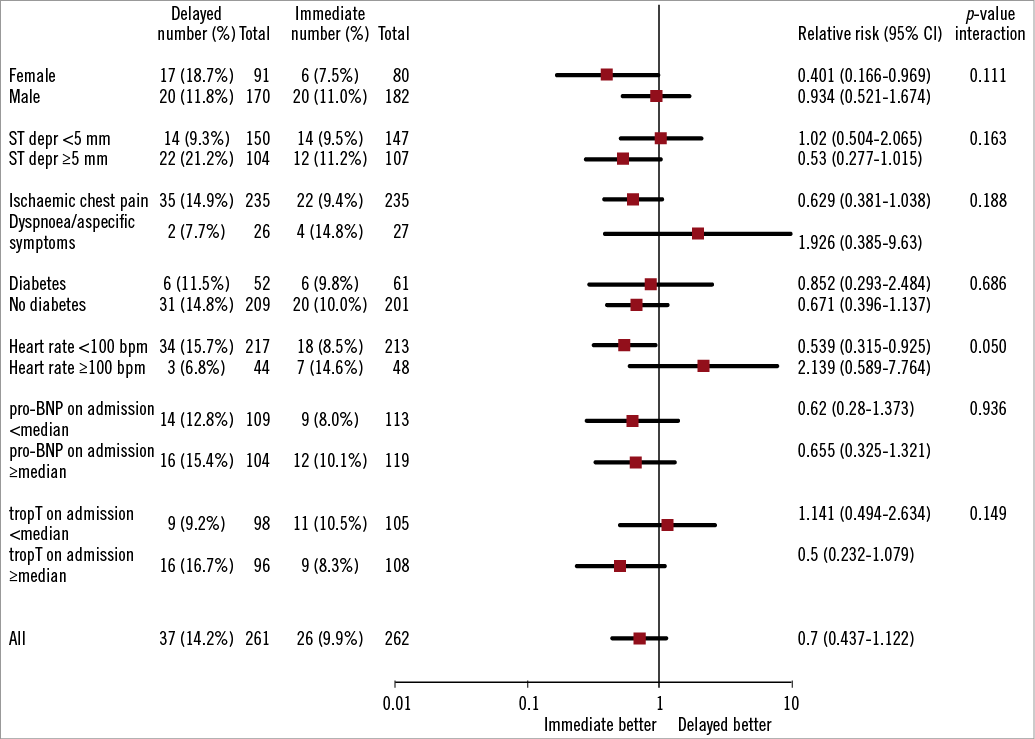
Figure 2. Forest plot of relative risk of primary endpoint at 30 days in pre-specified patient subgroups. Data are number or number (%), unless otherwise indicated. Percentages are number of patients with primary endpoint divided by number of patients. Squares and horizontal bars represent within-subgroup relative risk and 95% CIs, respectively, on a log scale.
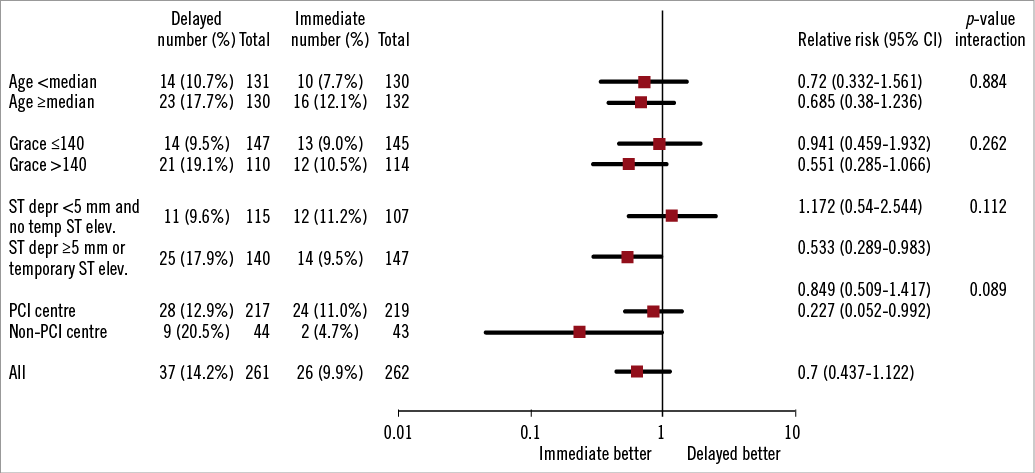
Figure 3. Forest plot of relative risk of primary endpoint at 30 days in post hoc selected patient subgroups. Data are number or number (%), unless otherwise indicated. Percentages are number of patients with primary endpoint divided by number of patients. Squares and horizontal bars represent within-subgroup relative risk and 95% CIs, respectively, on a log scale.
The first secondary endpoint, median enzymatic infarct size as assessed by a single cardiac troponin T measured at 72-96 hours after admission or at discharge, was similar in both groups (0.31 µg/l, p=0.98). The percentage of patients without a rise in CKMB during admission, the second secondary endpoint, was 35.4% in the immediate treatment group and 36.5% in the delayed treatment group (p=0.80).
INCIDENCE OF BLEEDING AND HOSPITAL STAY
No difference in the occurrence of bleeding events between the two treatment groups was found. Major bleeding occurred in 11.8% of the patients in the immediate treatment group and 11.1% in the delayed treatment group (p=0.80), while any bleeding was reported in 22.9% and 19.9% of the patients, respectively (p=0.41). Hospital stay for patients allocated to immediate treatment was significantly shorter than for patients in the delayed treatment group (median 4.0 vs. 6.0 days, p<0.001). The use of evidence-based medication at discharge was similar in both groups.
Discussion
The main finding of our study is that, in high-risk NSTE-ACS patients, angiography and revascularisation within 12 hours did not significantly improve clinical outcome as compared to intervention 48 hours or more after admission. Although we found a relative risk reduction (RRR) of about 30% for the combined endpoint of death, reinfarction or recurrent ischaemia at 30 days in the immediate group, this difference was not statistically significant. This difference was largely driven by a reduction in recurrent ischaemia (RRR=40%, p=0.058). Both secondary endpoints, enzymatic infarct size and the percentage of patients without a rise in CKMB during admission, were similar. This indicates that an early intervention does not lead to an increase in infarct size, as found by previous trials. Given this finding and the finding of similar rates of bleeding events, no difference in safety existed between the two strategies.
Of all trials investigating the optimal timing of intervention in high-risk NSTE-ACS patients, our study included patients with the highest median age (71.9 years). More than 70% of patients were above 65 years of age. As a result, this study reflects very well the age distribution of patients presenting with NSTE-ACS in real life, with a median age of 68 years20. A median GRACE risk score of 135 indicates that about half of the included patients had a high-risk profile.
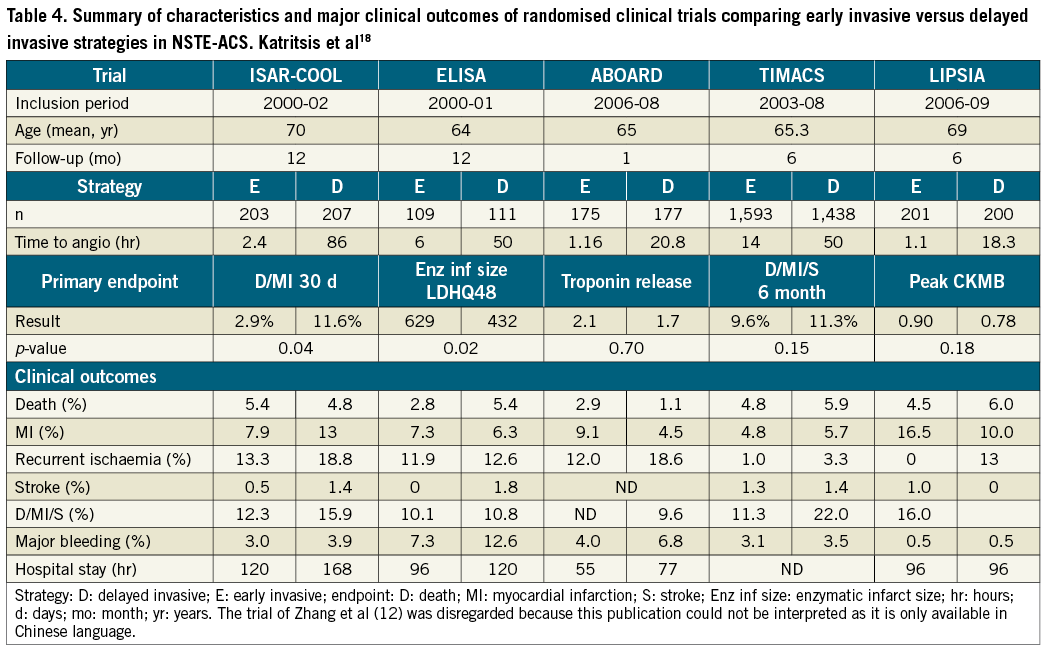
The possibility of comparing our results with those of previously published trials on this topic is limited due to differences in endpoints and timing of early and delayed intervention. Moreover, some trials took a non-clinical parameter as primary endpoint and clinical parameters only as secondary endpoints. However, taking these limitations into account, the results of our trial are largely consistent with randomised trials in Table 4 and with the conclusion of several meta-analyses17-19, and suggest that immediate intervention does not seem to be associated with a reduction in death or myocardial infarction, but at most reduces the incidence of recurrent ischaemia. However, a new meta-analysis should be performed. To address this question, this new meta-analysis should include the most recent trials.
The results of the subgroup analyses showed that patients at higher risk, as indicated by a higher GRACE risk score, a higher troponin level and/or more ST segment deviation deviation on admission, tend to benefit more from an early invasive strategy. These findings are in line with those of previously published trials and meta-analyses and support the most recent ESC guidelines for management of acute coronary syndromes in patients presenting without persistent ST-segment elevation1.
To our knowledge, this is the first trial which also recruited patients in non-PCI centres. For these centres, immediate angiography and intervention is not easy to perform on a 24/7 basis. Patients assigned to immediate intervention were transported immediately to the PCI centre by ambulance, whereas patients assigned to delayed intervention underwent angiography at their own centre. This resulted in a lower rate of ad hoc revascularisation (PCI) in this group further delaying revascularisation. Subgroup analysis showed that patients randomised at a non-PCI centre tended to benefit most from immediate intervention as compared to patients randomised at a PCI centre. This finding should be interpreted with caution but merits further investigation.
Although no definite conclusions can be drawn from this study with regard to the economic consequences of a routine early intervention strategy, a two-day shorter hospital stay might indicate a reduction in health costs. Extra costs for 24-hour a day, everyday availability of PCI facilities and emergency transportation of these patients by ambulance from hospitals without a primary PCI facility to an intervention centre, must be taken into account.
A major limitation of our study was the lower than expected incidence of the primary endpoint at 30-day follow-up. It was assumed to be 25% (see sample size calculation). After completion of the trial, however, this appeared to be considerably lower (14%), which means that the study was underpowered to prove the superiority of immediate intervention. Therefore, we cannot exclude that a larger number of included patients might have resulted in a different outcome. Another limitation in a trial of this kind is that the occurrence of periprocedural MIs is very difficult to assess accurately when patients undergo angioplasty at the moment of elevated cardiac enzymes, which may therefore result in a lack of detection of these MIs in patients who undergo immediate angioplasty. This is the reason why additional secondary endpoints concerning enzymatic infarct size during admission were assessed (troponin T at 72-96 hours after admission or at discharge, and the percentage of patients without a rise in CKMB during admission).
In summary, in this population of high-risk NSTE-ACS patients, we found that an immediate invasive strategy is safe and feasible, but not superior to a delayed invasive strategy in terms of prevention of the composite endpoint of death, reinfarction or recurrent ischaemia at 30-day follow-up. These results are consistent with previous trials and meta-analyses.
Acknowledgements
We would like to thank the staff in the coronary care units and the catheterisation laboratories and the cardiologists of the participating institutions. Furthermore, the help with the statistical analysis of Evelien Kolkman and Petra Koopmans from Diagram (Diagnostic Research and Management, Zwolle, The Netherlands) was very much appreciated.
Funding
Abbott (unrestricted research grant).
Conflict of interest statement
E. Badings received consulting fees from Merck Sharp and Dohme and Sanofi-Aventis. A. van ‘t Hof received speakers fees and research grants from Merck, Sanofi-Aventis, The Medicines Company, Iroko Cardio and AstraZeneca. The other authors have no conflicts of interest to declare.
Appendix
Investigators who recruited at least one patient:
– Isala Klinieken, Zwolle: A.W.J. van ’t Hof, J.H.E. Dambrink, J. Timmer;
– Ziekenhuis Bethesda, Hoogeveen: S.H.K. The, R.J.M. de Vries, M.L.J. van der Wielen, R.F. van Es, E.H. Hoekstra;
– Deventer Ziekenhuis, Deventer: J. van Wijngaarden, D.J.A. Lok, E. Badings;
– Scheper Ziekenhuis, Emmen: L.M.F. van den Merkhof, M.W.J. Vet;
– Ziekenhuis De Tjongerschans, Heerenveen: G. Tjeerdsma, M. Kooistra, J.S. van Os, G.M. Jochemsen

