Abstract
Background: Current guidelines stress the importance of early invasive assessment of patients with non-ST-elevation acute coronary syndrome (NSTE-ACS), in particular those at high risk. However, supporting scientific evidence is limited.
Aims: We aimed to investigate the prognostic impact of the timing of coronary angiography in a large cohort of NSTE-ACS patients.
Methods: We performed a retrospective analysis including 34,666 NSTE-ACS patients registered from 2013 to 2018 in the SWEDEHEART registry. The prognostic implications of the timing of coronary angiography on a continuous scale and within <24 vs 24-72 hours were assessed using Cox regression analyses.
Results: The median time interval from admission to invasive assessment was 32.8 (25th, 75th percentiles 20.4-63.8) hours. There was no apparent time window within 96 hours from admission that provided prognostic benefit. Coronary angiography within 24-72 hours (vs <24 hours) was not associated with worse outcome overall (all-cause mortality: hazard ratio 1.01, 95% confidence interval [CI] 0.92-1.11; major adverse events: hazard ratio 1.04, 95% CI: 0.98-1.12). Interaction analyses indicated a greater relative benefit of coronary angiography <24 hours in some lower-risk groups (women, non-diabetics, patients with minor troponin elevation) but neutral effects in higher-risk groups (defined by age or the GRACE 2.0 score).
Conclusions: These Swedish data do not provide support for an early invasive strategy in NSTE-ACS, especially in high-risk patients. Our results suggest that the timing of invasive assessment should rather be based on individualised decisions integrating symptoms and risk panorama than on strictly defined time intervals.
Introduction
Current guidelines recommend early invasive assessment in patients with non-ST-elevation acute coronary syndrome (NSTE-ACS) with the intention to identify and treat critical coronary lesions12. An invasive approach has consistently been shown to reduce the occurrence of ischaemic outcomes, particularly in patients at high risk345. Immediate (<2 hours) coronary angiography is recommended in haemodynamically unstable patients, those with cardiogenic shock, and those with other life-threatening complications or refractory/recurrent angina. High-risk patients, defined by dynamic ST-changes, and patients with a high Global Registry of Acute Coronary Events (GRACE) score or cardiac troponin changes should be investigated within <24 hours12. The 2020 European guidelines have extended this recommendation to all patients with non-ST-elevation myocardial infarction (NSTEMI)1. In the remaining NSTE-ACS patients, selective invasive assessment, e.g., within 24-72 hours, is regarded as optimal12.
These recommendations are based on conceptual considerations and the results from the TIMACS and VERDICT studies, which randomised moderately sized NSTE-ACS cohorts to early (within 12-24 hours) or delayed (after ≥36 hours6; within 48-72 hours7) angiography. Support for these recommendations comes from post hoc analyses of clinical trials89 and meta-analyses101112. Prospective studies were, however, underpowered with respect to single, clinically relevant outcomes. TIMACS, moreover, was published more than a decade ago, and real-world patients may differ considerably to NSTE-ACS patients recruited in randomised controlled trials in terms of higher age and higher prevalence of comorbidities.
Previous results from the SWEDEHEART (Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies) registry demonstrated that percutaneous coronary intervention is associated with prognostic benefit regardless of when it is performed during the first 3 days of hospitalisation13. That study was based on NSTEMI patients admitted between 2006 and 2013. In the present investigation, we extend these data to a more contemporary cohort of NSTE-ACS patients, hospitalised from 2013 to 2018, with granular information on the timing of the invasive assessment. Our aims were to investigate the prognostic implications of the time to coronary angiography, both overall, in predefined at-risk cohorts, and with the intention of identifying a time window that provides particular benefits regarding outcome.
Methods
Study population
This study is part of the TOTAL-AMI (Tailoring Of Treatment in All comers with Acute Myocardial Infarction) project. The primary aim of TOTAL-AMI is to investigate the mechanisms and implications of different myocardial infarction (MI) subtypes14 and of comorbidities (e.g., chronic obstructive pulmonary disease, atrial fibrillation, renal dysfunction) in MI. TOTAL-AMI uses data from SWEDEHEART, which is a registry aggregating information on consecutive patients admitted to Swedish coronary care units or other specialised facilities because of suspected acute coronary syndrome. SWEDEHEART provides nationwide coverage and lifelong follow-up. Upon hospital admission, patients receive information about the registry, have the right to deny participation and have their data erased upon request. Written informed consent is not required according to Swedish law.
For the present analysis, all NSTE-ACS patients hospitalised between January 2013 and May 2018 were considered. Only first-time admissions during the study period were counted. The diagnoses of unstable angina and NSTEMI had been set by the attending clinicians at each respective hospital. Since decisions regarding the performance and timing of coronary angiography might have been affected by the presence of specific comorbidities, patients with dementia, haemoglobin <80 g/L and an estimated glomerular filtration rate (CKD-EPI equation) <20 mL/min/1.73 m2 were excluded. Moreover, we excluded patients presenting with cardiogenic shock, cardiac arrest or following prehospital resuscitation since immediate invasive assessment may be warranted in these groups. Finally, patients with outlying values for the time to coronary angiography15 were excluded.
All data were made pseudonymised before the statistical analyses. The study was conducted according to the principles of the 1975 Declaration of Helsinki and was approved by the Regional Ethical Review Board in Stockholm (2012/60-31/2).
Timing of coronary angiography
Time to coronary angiography was defined as the time interval from admission to the emergency department or coronary care unit, whichever came first, to arterial puncture at the cath lab. The time interval is given in the dataset in minutes and was considered both on a continuous scale and categorised by time intervals focusing on 24 hours as the threshold12.
Prognostic evaluation
Information on patient outcome was obtained by merging SWEDEHEART with data from the Swedish Population registry (data on the vital status of all Swedish residents) and the mandatory Swedish Patient Registry (hospitalisation dates and discharge diagnoses based on International Classification of Diseases, 10th revision, Clinical Modification [ICD-10-CM] codes), the latter held by the Swedish Board of Health and Welfare. The main outcomes considered for this analysis were all-cause mortality with available data until May 2018, and major adverse events (MAE) with available data until December 2017. MAE was defined as the composite of all-cause mortality, hospitalisation for MI (ICD-10-CM I21), heart failure (ICD-10-CM I50) or ischaemic stroke (ICD-10-CM I63). During the first 30 days after the index hospitalisation, it is not possible to separate a new MI from the index MI in the patient registry. Therefore, only MI occurring at least 30 days after the index hospitalisation were counted to avoid “contamination” from the index event. Differences in follow-up periods are due to a time lag for the patient registry to process hospitalisation data and make them accessible. As a safety outcome, we also assessed bleeding, defined as major bleeding during the index hospitalisation or readmission with a bleeding-related primary diagnosis (Supplementary Table 1) until December 2017.
Statistical analysis
We used the following approach to investigate the prognostic implications of the time to coronary angiography. First, Cox regressions were conducted, modelling time as a continuous variable and per 24-hour intervals. The intention of this part of the analysis was to use an approach that was strictly data driven. Analyses were adjusted for admission year, hospital, time and date of admission (outside office hours, on weekends or public holidays), age, sex, current smoking, hypertension, diabetes mellitus, hyperlipidaemia, previous MI, previous coronary revascularisation, previous heart failure, previous stroke, ST-segment changes on the admission electrocardiogram (ECG), atrial fibrillation on the admission ECG, estimated glomerular filtration rate, chronic obstructive pulmonary disease, previous or present cancer and peripheral vascular disease. Due to positive skew, time was ln-transformed before being entered into the analyses, when appropriate. The proportional hazard assumptions had been checked by visual inspection of the log-cumulative hazard plots and were found to be satisfied. Risk estimates are described as hazard ratios (HR) with 95% confidence intervals (CI).
Next, we intended to more directly address the recommendations issued by current guidelines12. For this purpose, we compared outcomes associated with coronary angiography at <24 vs 24-72 hours. Patients who had died within 72 hours from admission were censored in order to avoid immortal time bias. As a sensitivity analysis, this step was repeated in 1:1 propensity score-matched patients based on the same set of covariates as used for the Cox regressions. Secondary subgroup analyses with interaction testing were conducted in cohorts defined by sex, age tertiles, diabetes, cardiac troponin concentrations (≤99th percentile, and divided by the median ratio to the 99th percentile for those with concentrations >99th percentile), and low, intermediate or high risk according to the GRACE 2.0 score1617. Kaplan-Meier curves were constructed to visualise the cumulative incidence of all-cause mortality and MAE in selected subgroups.
Continuous variables are reported as medians with 25th and 75th percentiles. Categorical variables are expressed as frequencies and percentages. In all tests, a two-sided p-value <0.05 without correction for multiple testing was considered significant. The software packages SPSS 27.0 (SPSS Inc.) and R version 4.0.5 (R Foundation for Statistical Computing) were used for the analyses.
Results
In total 44,366 NSTE-ACS patients had been registered in SWEDEHEART between January 2013 and May 2018. Information on the time from admission to coronary angiography was available in 39,571 patients. Following exclusions (Supplementary Figure 1), 34,666 patients formed the final study cohort. The median age was 70 (62-77 [25th, 75th percentiles]) years and 23,906 (69.0%) patients were male. The median time interval to angiography was 32.8 (20.4-63.8) hours. In total, 25,579 (73.8%) patients underwent in-hospital coronary revascularisation and 27,688 (79.9%) patients were discharged with a diagnosis of NSTEMI. Further information on clinical characteristics is presented in Table 1.
Over a median follow-up of 2.4 (1.2-3.8) years, 3,078 (8.9%) patients died. The median follow-up regarding MAE was 2.0 (0.8-3.3) years. During this time, 3,027 (9.3%) patients died, 1,663 (4.8%) patients suffered an MI, 1,551 (4.5%) patients were readmitted due to heart failure and 569 (1.6%) patients were readmitted due to stroke. In total 5,231 (15.1%) patients had an MAE. A bleeding event occurred in 1,179 (3.4%) patients.
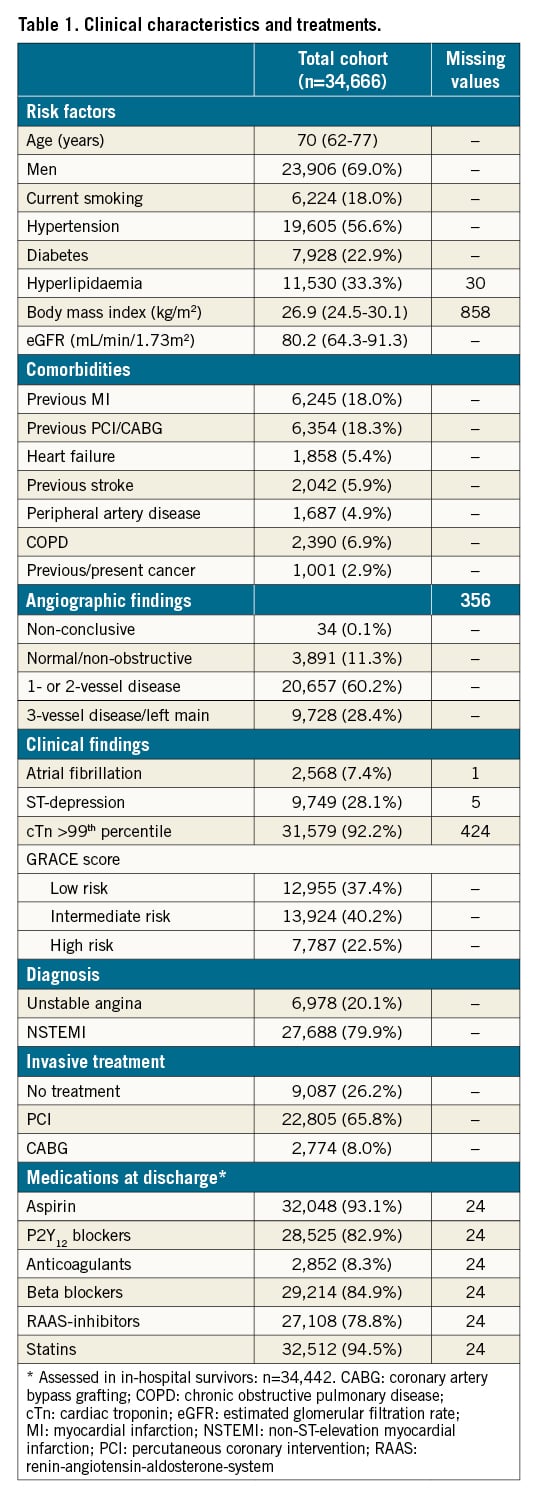
Crude rates of all-cause mortality and MAE increased across greater 24-hour intervals to coronary angiography (Supplementary Figure 2). In a fully adjusted Cox model, a 1-standard deviation increase in the ln-transformed time interval was associated with 4% risk increases regarding both all-cause mortality and MAE at borderline levels of significance (Table 2). The association with MAE was driven by a significant 11% risk increase regarding heart failure hospitalisations. The associations of the time interval with MI, stroke or bleeding were neutral. Using NSTE-ACS patients assessed at <24 hours as reference, delayed coronary angiography exhibited similar associations with all-cause mortality and MAE, unless performed late, i.e., after 96 hours (Figure 1). Patients assessed late were older, more often female, had more cardiovascular comorbidities but also less often had troponin elevation (Supplementary Table 2).
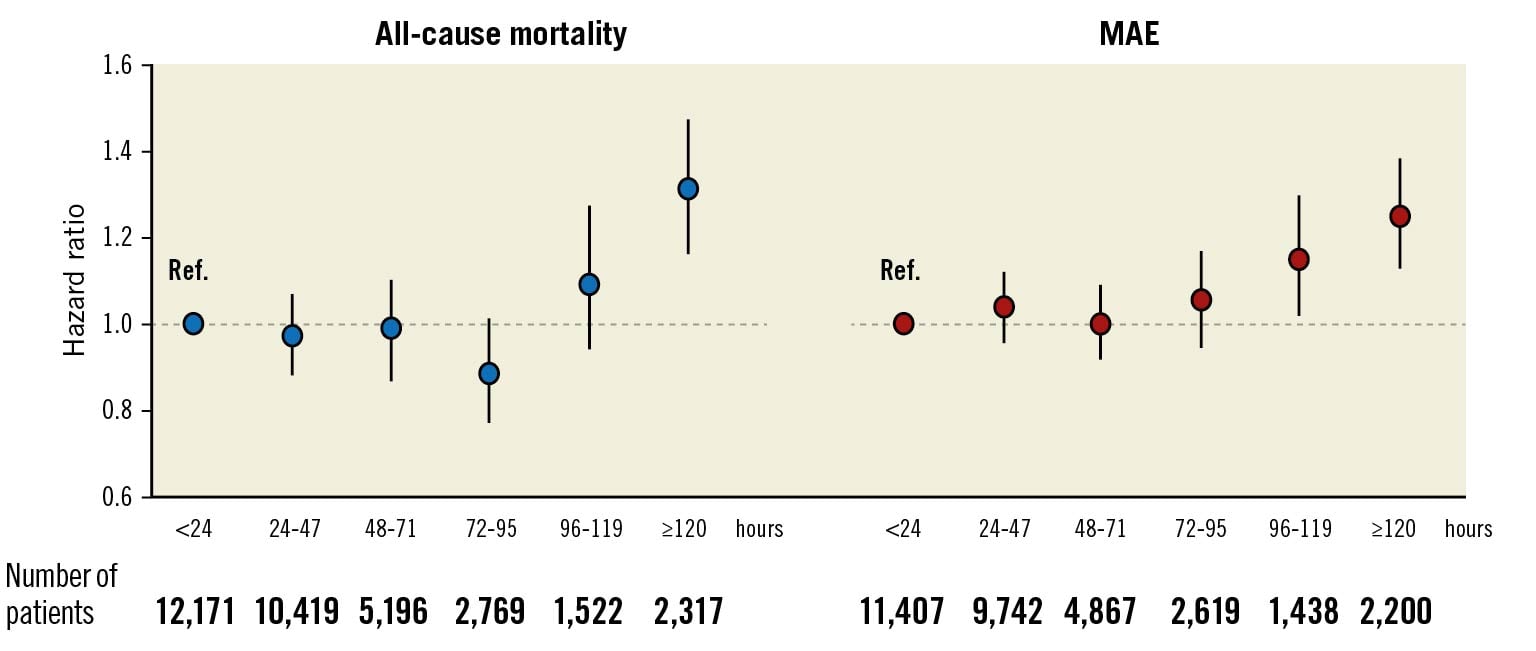
Figure 1. Association of increasing time intervals to coronary angiography with adverse outcome. Adjusted for admission year, hospital, time and date of admission, age, sex, current smoking, hypertension, diabetes, hyperlipidaemia, previous myocardial infarction, previous coronary revascularisation, congestive heart failure, previous stroke, ST-changes upon admission, atrial fibrillation upon admission, estimated glomerular filtration rate, chronic obstructive pulmonary disease, previous or present cancer and peripheral vascular disease. MAE: major adverse event; Ref: reference.
In our next step, we investigated outcomes in 3-day survivors who underwent coronary angiography within <24 vs 24-72 hours (n=27,888). Patients assessed within <24 hours tended to be younger, were more often male, had a lower prevalence of cardiovascular risk factors and comorbidities, and more often had troponin elevation and lower GRACE 2.0 scores. Moreover, crude event rates were lower in this cohort (Supplementary Table 3). However, compared to these patients, those assessed within 24-72 hours had similar risks of all-cause mortality or MAE upon multivariable adjustment (Table 3, Central illustration). We noted a 12% increased risk of MI, albeit at a borderline level of significance (HR 1.12, 95% CI: 1.00-1.26; p=0.052). The adjusted risks for the other assessed outcomes, including bleeding, did not differ statistically between both cohorts (data not shown). The sensitivity analysis based on 1:1 propensity score-matched patients provided similar results with respect to all-cause mortality and MAE, as indicated by largely overlapping CI compared to the Cox models (Table 3). Secondary subgroup analyses with interaction testing indicated greater all-cause mortality risk associated with coronary angiography within 24-72 hours in some lower-risk cohorts, i.e., patients with minor troponin elevation and non-diabetics (Central illustration). For women, coronary angiography within 24-72 hours was associated with increased risk of MAE. The cumulative incidence of adverse outcome in these cohorts is presented in the Supplementary Figures 3A-Supplementary Figure 3C. The interactions within the other subgroups were neutral.
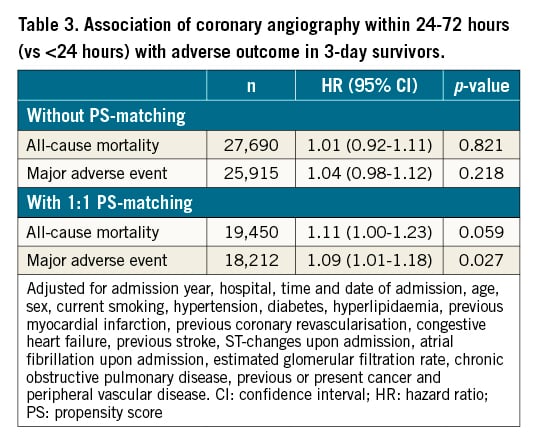
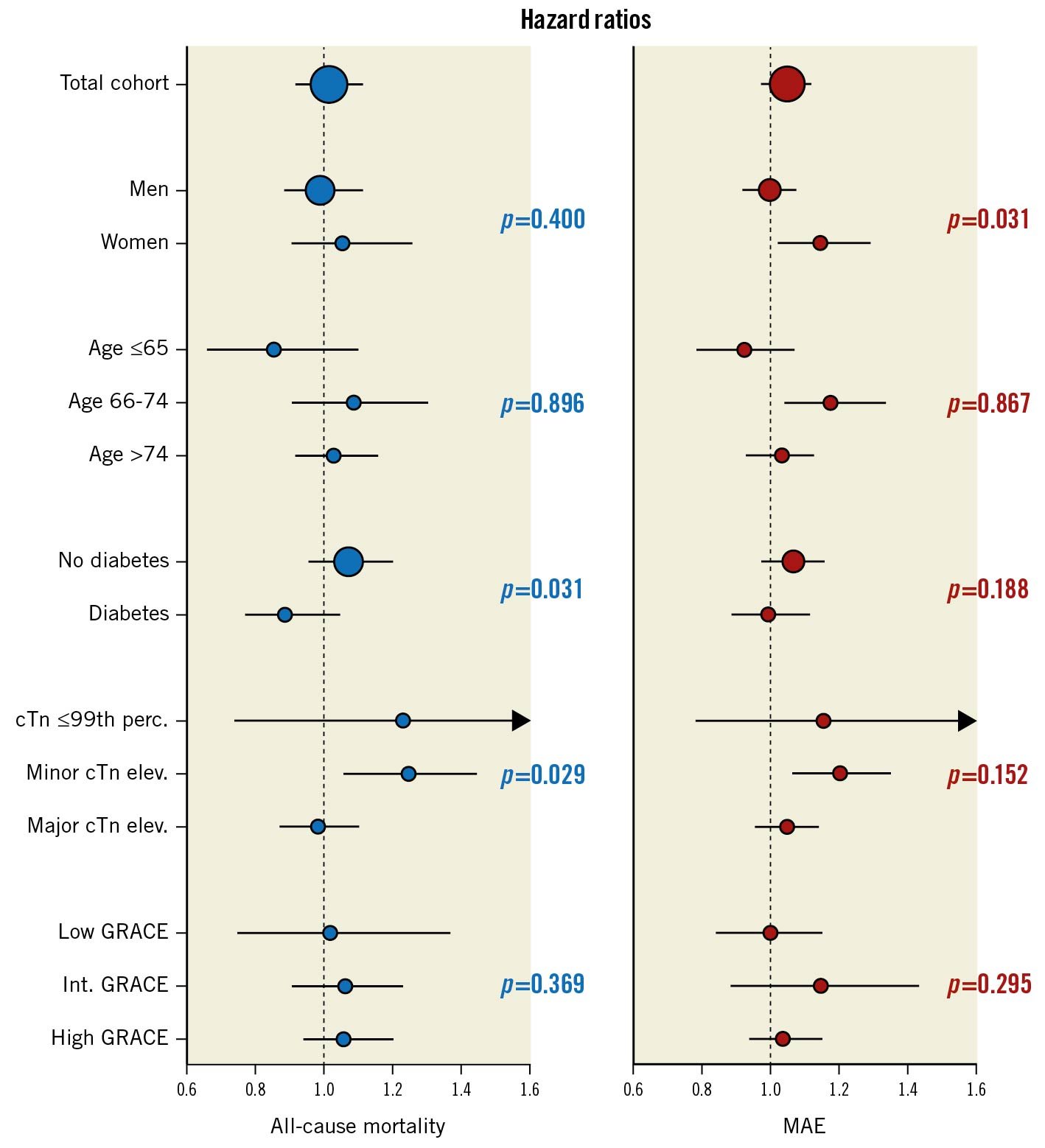
Central illustration. Association of coronary angiography within 24-72 hours (vs <24 hours) with adverse outcome in 3-day survivors overall and in subgroups. P-values refer to the interactions of subgroup categories on the associations of the time to coronary angiography with adverse outcome. Adjusted for admission year, hospital, time and date of admission, age, sex, current smoking, hypertension, diabetes, hyperlipidaemia, previous myocardial infarction, previous coronary revascularisation, congestive heart failure, previous stroke, ST-changes upon admission, atrial fibrillation upon admission, estimated glomerular filtration rate, chronic obstructive pulmonary disease, previous or present cancer, peripheral vascular disease, as appropriate. cTn: cardiac troponin; MAE: major adverse event; perc: percentile.
Discussion
It is well established that early coronary reperfusion improves outcome in ST-elevation MI where the infarct-related artery is usually occluded. However, the optimal timing of coronary angiography is less clearly defined in NSTE-ACS, a condition characterised by sudden plaque disruption with subocclusive thrombus formation and distal embolisation of thrombotic debris.
Our analysis investigating a large cohort of NSTE-ACS patients provides new evidence in this regard. A longer time interval to coronary angiography was associated with a 4% increased risk of all-cause mortality and MAE. These risk gradients were driven by patients assessed late, i.e., after 96 hours, being characterised by a greater burden of comorbidities. Accordingly, clinicians may have been primarily inclined to target these patients for a conservative approach, thus delaying time to angiography. Interestingly, patients investigated after 96 hours less often had elevated troponin concentrations and, for this reason, may not have been considered as being in need of early invasive assessment. Importantly however, we were unable to identify a time window within the first 96 hours from admission that provided a particular prognostic benefit.
The rationale behind early invasive assessment in NSTE-ACS is the intention to identify and treat critical coronary lesions early in the disease course. This may limit infarct size and prevent recurrent ischaemia, thereby promoting clinical stabilisation, improving the well-being of the individual patient and facilitating earlier discharge. Scientific evidence on the prognostic implications of early coronary angiography in NSTE-ACS is, however, conflicting. Some studies reported neutral results regarding hard outcomes671819, whereas others provided evidence supporting early8913 or delayed20 assessment. Based on subgroup analyses from randomised trials and meta-analyses67101112, and conceptual considerations, current European and US guidelines recommend coronary angiography within <24 hours in all patients with a perceived increased risk (i.e., defined by intercurrent ST-changes or GRACE score >140 using a previous iteration of this tool12), provided there is an absence of high-risk features such as refractory/recurrent ischaemia or haemodynamic instability. US guidelines also advocate early assessment in patients with dynamic troponin changes2, whereas recently published ESC guidelines have extended this recommendation to all patients with NSTEMI1.
Our data provide a differentiated perspective on this important topic. Overall, early coronary angiography was neither associated with improved outcome nor with harm. Risk reductions were noted in some lower-risk cohorts, i.e., patients with minor troponin elevation and also women. Even for non-diabetics, interaction analysis indicated a greater relative benefit with early assessment. However, we found no evidence indicating that angiography within <24 hours might be associated with better outcome in high-risk cohorts. This was not explained by an excess in bleeding risk in the case of delayed assessment.
These data challenge the necessity of early coronary angiography in high-risk patients, for example those with a GRACE score >1402. There has been ambiguity on this matter since this recommendation is only based on subgroup analyses from TIMACS and VERDICT67. Recently published real-world data provided no supporting evidence19, and the optimal timing in relation to the GRACE 2.0 score remains debated2122. The ongoing RapidNSTEMI trial (ClinicalTrials.gov identifier: NCT03707314) will hopefully provide clarification. The lack of prognostic benefit by earlier coronary angiography in patients with major troponin elevation was unexpected since the magnitude of troponin release is associated with the severity of coronary lesions in NSTE-ACS23. Further mechanistic analyses are needed to study the interrelationship of invasive assessment, its timing, troponin concentrations and outcome. There is also a need for further in-depth exploration of the prognostic implications of the timing of coronary angiography in relation to sex.
Strengths and limitations
Our study has several strengths. The investigated cohort was large and had longer follow-up compared to those assessed in previous trials and meta-analyses6789101112181920. SWEDEHEART is a real-world registry covering all hospitalisations in Sweden due to suspected acute coronary syndrome. This offers the advantage of investigating outcomes and subcohorts that may be underrepresented in randomised controlled trials. There are also some limitations that need to be considered. Although all hospitals participating in SWEDEHEART are annually monitored, the data cannot be of the same quality as in a prospective study. However, the accuracy of the data and the registry have been found to be high24. Our results may have been affected by unmeasured confounders. For example, SWEDEHEART does not capture all data surrounding the timing of invasive assessment. Decisions towards an earlier approach may have been triggered in some patients by signs of coronary instability, e.g., recurrence of ischaemic symptoms or intercurrent ECG changes not documented in SWEDEHEART. Timing decisions could also have been delayed by logistical problems such as long transportation distances or limitations in available hospital beds, by patient refusal, comorbidities or frailty. This could have contributed to the high event rates in patients assessed very late. We acknowledge that the timing of coronary angiography most likely has different prognostic implications in type 1 and type 2 MI, the latter often characterised by stable coronary lesions. We were not able to consider this issue specifically since data on the MI type is incomplete in SWEDEHEART. MI occurring early (<30 days) after hospital discharge could not be studied since such information is not available in the Swedish Patient Registry. However, previous studies found no evidence of increased MI risk within 15-30 days in NSTE-ACS patients undergoing early coronary angiography67. We lack information on periprocedural MI and softer endpoints such as recurrent ischaemia after discharge. We did not account for the prognostic effects of methodological improvements during the observation period, e.g., the use of newer-generation drug-eluting stents or different regimes of antiplatelet therapy. Finally, caution is warranted when inferring causality on the observed associations.
Conclusions
Whether or not patients with NSTE-ACS may benefit from early invasive assessment has been a matter of debate for a long time. Our findings demonstrate that an invasive approach within <24 hours is not associated with improved outcome apart from for some lower-risk groups in whom there were indications of risk reduction. Therefore, the timing of invasive assessment should be based rather on individualised decisions integrating symptoms, risk panorama and available resources than on strictly defined time intervals.
Impact on daily practice
The present study demonstrates that early invasive assessment has limited impact on outcome in patients with NSTE-ACS. Earlier coronary angiography, e.g., within <24 hours, may expedite patient management towards earlier discharge but places logistical demands on the healthcare system. A delayed approach seems to be at least as safe provided there is clinical stability. Our data suggest that invasive assessment should be based on individualised decisions integrating symptoms, risk panorama and available resources rather than on strictly defined time intervals.
Acknowledgements
We wish to express our gratitude to Lars Lindhagen at Uppsala Clinical Research Center for statistical support.
Funding
The TOTAL-AMI project has received funding from the Swedish Foundation of Strategic Research. This organisation had no role in the collection, analysis or interpretation of the data, in the writing of the report or in the discussion to submit this paper for publication.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

