Abstract
Aims: We sought to describe the incidence, predictors, and impact of adverse neurological events (NE) in a non-ST-segment elevation acute coronary syndromes (NSTEACS) population undergoing percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), or medical therapy (MT).
Methods and results: 13,819 patients with moderate and high-risk NSTEACS were enrolled in the prospective ACUITY trial. Angiography was performed within 72 hours of presentation, after which 7,789 patients (56.4%) underwent PCI, 1,539 (11.1%) underwent CABG, and 4,491 (32.5%) received MT. The rate of NE (stroke or transient ischaemic attack) at 30 days and one year and its relationship to adverse ischaemic events, including death, were assessed. Thirty-day rates of NE were 1.1% with CABG, 0.3% with PCI, and 0.5% with MT (p<0.001). One-year rates of NE were 1.1% with CABG, 0.3% with PCI, and 0.6% with MT (p<0.001). Independent predictors of NE at 30 days and one year included age, renal insufficiency, baseline troponin elevation, and initial treatment with CABG. The occurrence of NE was a strong independent predictor of death at 30 days and one year (HR 4.07, 95% CI [1.49, 11.11], p=0.006, and HR 4.25, 95% CI [2.37, 7.62], p<0.001, respectively).
Conclusions: In the large-scale ACUITY trial, CABG was associated with a higher risk of NE at 30 days and one year compared to PCI and MT. The occurrence of NE in patients with NSTEACS was strongly associated with increased early and late mortality. Clinical Trials.gov Identifier NCT00093158
Introduction
The occurrence of stroke in patients with non-ST-segment elevation acute coronary syndromes (NSTEACS) is uncommon but potentially devastating. Multiple studies have evaluated this outcome, including the SYNTAX and FREEDOM trials, which noted higher incidences of neurological events (NE) in CABG compared to PCI at less than and beyond one year1-3. However, both examined patients with high degrees of coronary artery disease rather than patients with active NSTEACS. Revascularisation with PCI or CABG utilising newer anticoagulants such as bivalirudin has yet to be examined in this NSTEACS population. Furthermore, whether revascularisation at all carries a greater risk than medical therapy (MT) has not been described. We therefore sought to evaluate the incidence and predictors of NE in moderate to high-risk NSTEACS patients in the large-scale, multicentre, prospective, randomised ACUITY (Acute Catheterisation and Urgent Intervention Triage StrategY) trial.
Methods
PATIENTS AND STUDY PROTOCOL
The ACUITY trial design has been previously reported in detail4. Briefly, ACUITY was a multicentre, prospective, randomised trial of 13,819 patients with moderate- and high-risk NSTEACS who were managed with an early invasive strategy. While the inclusion and exclusion criteria have been previously described, of note, patients with arteriovenous malformations, cerebral aneurysm, prior ischaemic stroke within the last two years, prior haemorrhagic stroke or stroke complicated by residual neurologic deficits were ineligible for enrolment. Patients were randomly assigned prior to coronary angiography to heparin (unfractionated or low-molecular-weight) plus a glycoprotein IIb/IIIa inhibitor, bivalirudin plus a glycoprotein IIb/IIIa inhibitor, or bivalirudin monotherapy with provisional glycoprotein IIb/IIIa inhibitor use. Angiography was performed in all patients within 72 hours of randomisation. Depending on the coronary anatomy, patients were subsequently treated with PCI, CABG, or MT. In patients undergoing PCI, the choice of using either bare metal or drug-eluting stents was left to the discretion of the operator. Dual antiplatelet therapy with aspirin and clopidogrel was strongly recommended for at least one year following PCI and with MT (unless normal coronary arteries were found). Aspirin was mandatory after CABG, but clopidogrel use was discretionary. All major adverse events were adjudicated by an independent clinical events committee blinded to treatment assignment and procedural outcomes. The occurrence of stroke and transient ischaemic attack (TIA) was collected in the case report form and these were site-reported events. Stroke and TIA were defined as new focal neurologic deficits lasting ≥24 hours and <24 hours, respectively.
Objectives
The primary objective of the present analysis was to evaluate the occurrence of NE in the study population and in each of the three treatment arms (PCI, CABG, and MT), and to evaluate its association with adverse ischaemic outcomes, in particular all-cause death.
Definitions and endpoints
NE was defined as any stroke or TIA occurring after randomisation. Composite major adverse cardiovascular events (MACE) were defined as death from any cause, myocardial infarction (MI), or unplanned revascularisation for ischaemia4. Endpoints were measured at 30 days and one year. Quantitative and qualitative coronary analysis was performed by an independent angiographic core laboratory (Cardiovascular Research Foundation, New York, NY, USA).
Statistical analysis
Categorical variables were compared with the chi-square test or Fisher’s exact test. Continuous variables were expressed as median and interquartile range and were compared using the Wilcoxon rank-sum test. Kaplan-Meier methods were used to estimate and plot time-to-event rates with comparisons made using the log-rank test. Cox proportional hazards regression was performed to identify the independent predictors of NE. The multivariable models were built by stepwise variable selection with entry and exit criteria set at the p<0.1 level. The number of variables was limited to avoid model overfitting. Based on prior studies5-9 and/or if judged clinically relevant, the covariates included were age, gender, weight, renal insufficiency, enoxaparin post randomisation, previous CABG, baseline troponin elevation, and CABG versus PCI or MT so as to avoid model overfitting. The relationship of NE to subsequent ischaemic events and death was analysed in a time-adjusted multivariable model. A two-sided p-value <0.05 was considered statistically significant for all analyses.
Results
Of 13,819 patients with moderate- and high-risk NSTEACS enrolled in the ACUITY trial, 7,789 patients (56.4%) underwent PCI, 1,539 (11.1%) underwent CABG (1,318 on-pump vs. 221 off-pump), and 4,491 (32.5%) were treated with MT alone. NE at 30 days and one year occurred in 59 (0.4%) patients and 68 (0.5%) patients, respectively. Among the 68 NE occurring within one year, 55 (80.9%) were defined as stroke and 13 (19.1%) were classified as TIA.
STUDY POPULATION
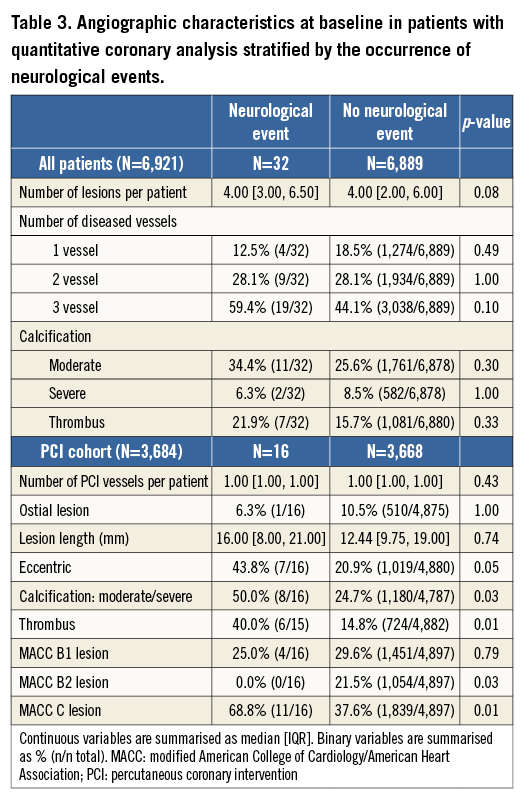
Baseline characteristics of patients with and without NE are shown in Table 1, Table 2 and Table 3. Patients with NE were generally older, female, were more likely to have lower weights, and had higher rates of baseline renal insufficiency, biomarker elevation, ST-segment deviation, higher TIMI risk scores, and higher baseline CHADS2 and CHA2DS2-VASc scores. Patients with NE were also more likely to have higher platelet and white blood cell counts, lower haemoglobin, and higher C-reactive protein levels at randomisation. NE patients were less likely to receive aspirin and/or a thienopyridine at discharge and were also significantly less likely to receive aspirin at one year. However, no significant differences in antiplatelet agent use were present at 30 days in patients with or without NE. Patients with NE were more likely to have received enoxaparin as the antithrombotic regimen after randomisation, while NE was less frequent in patients in whom bivalirudin was used. Among the 6,921 patients in whom QCA was performed as part of a formal pre-specified ACUITY substudy4, those who underwent PCI in whom NE developed had a greater frequency of modified ACC classification type C lesions, thrombotic lesions, eccentric plaque, and greater calcium burden.
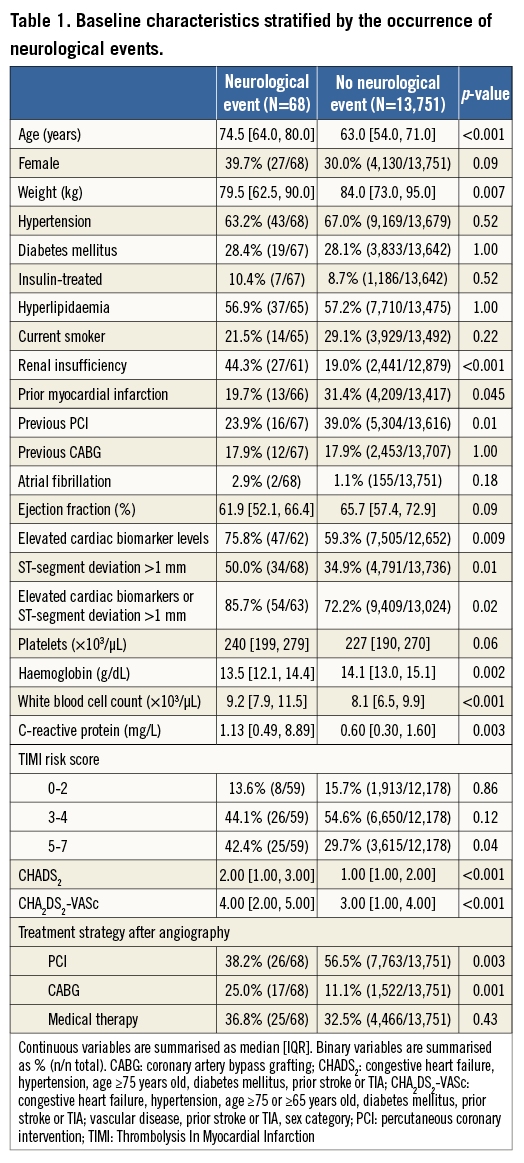
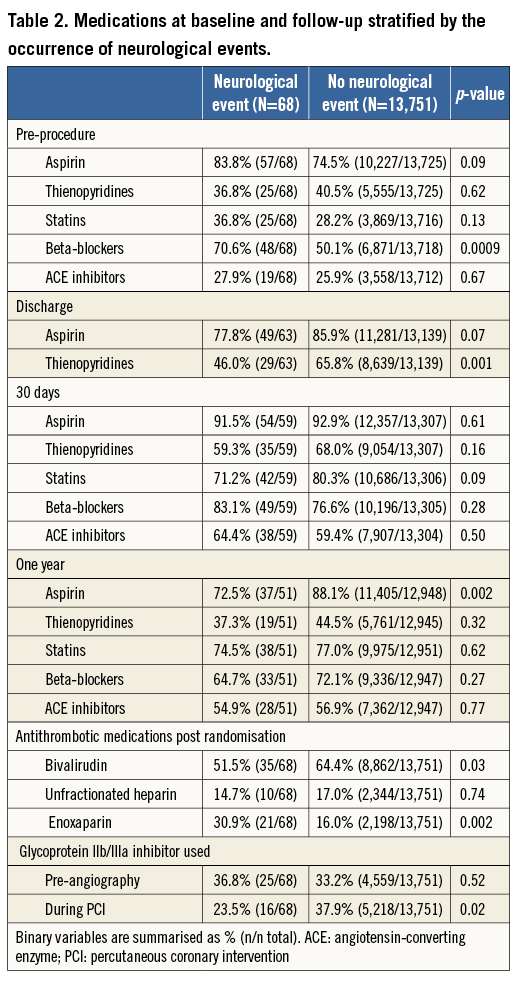
NEUROLOGICAL EVENTS ACCORDING TO TREATMENT STRATEGY
At 30 days the rates of NE were 1.1% with CABG, 0.3% with PCI, and 0.5% with MT (p<0.001), and at one year the respective rates of NE were 1.1%, 0.3%, and 0.6% (p<0.001) (Figure 1). The increased rate of events with CABG principally occurred within 30 days of surgery; thereafter, the rates of NE were similar among the three treatments (Figure 2). TIA represented four of the 17 events (23.5%) in the CABG group, four of the 26 events (15.4%) in the PCI group, and five of the 25 events (20%) in the MT group. Most NE occurred early after randomisation, typically post procedure (in the case of PCI and CABG) and before hospital discharge. The median (IQR) time to NE after randomisation was two (1,8) days after PCI, five (3,10) days after CABG, and six (3,13) days in the MT group, (p for trend=0.01). NE was first documented during or on the day of the procedure in four of the 17 patients with events after CABG (23.5%), in nine of the 26 patients (34.6%) with events after PCI, and in four of the 25 patients (16.0%) with events in the MT group (after angiography). All NE after CABG occurred in patients whose surgery was performed on-pump (17/1,318 [1.3%] vs. 0/221 [0%] with off-pump surgery, p=0.16).
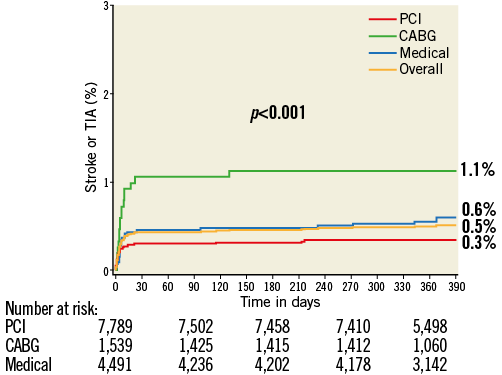
Figure 1. Kaplan-Meier curves showing cumulative event rates of stroke or TIA through one year.
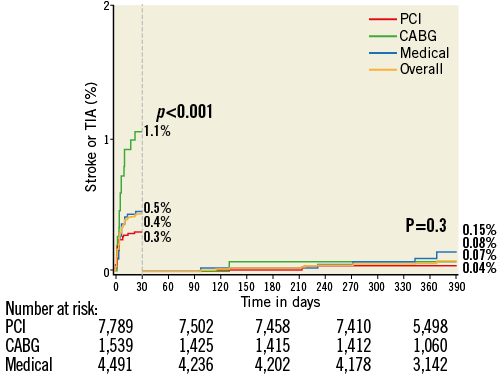
Figure 2. Kaplan-Meier curves showing cumulative event rates of stroke or TIA through one year with landmark analysis at 30 days.
INDEPENDENT PREDICTORS AND IMPACT OF NEUROLOGICAL EVENTS
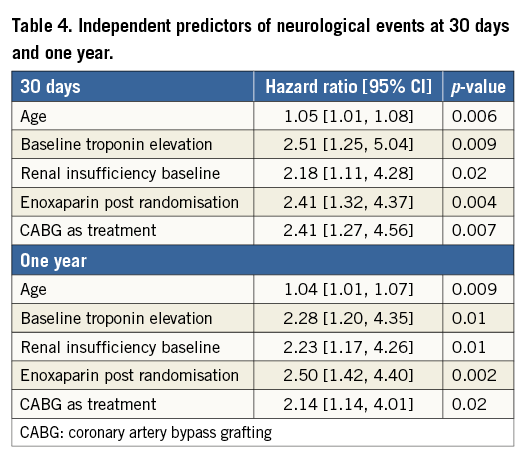
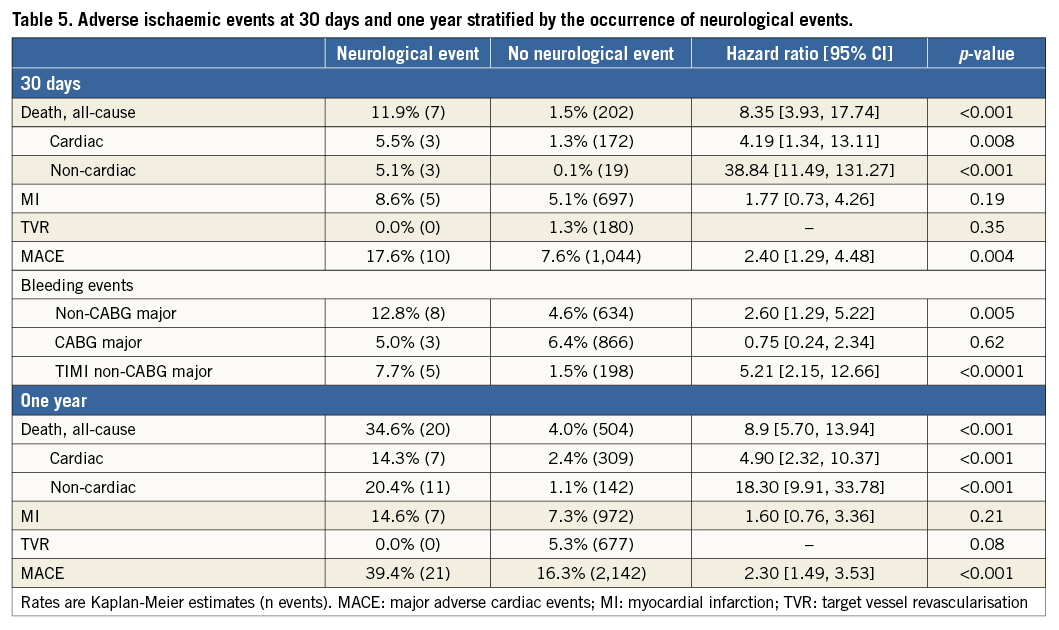
By multivariable analysis, age, baseline troponin elevation, baseline renal insufficiency, enoxaparin post randomisation, and treatment with CABG were independent predictors of NE at 30 days and one year (Table 4). Patients with NE compared to those without NE had significantly greater rates of MACE at 30 days (17.6% vs. 7.6%, HR 2.40, 95% CI [1.29, 4.48], p=0.004) and one year (39.4% vs. 16.3%, HR 2.30, 95% CI [1.25, 4.11], p<0.001) (Table 5). Death from any cause was markedly greater in patients with NE at 30 days (11.9% vs. 1.5%, HR 8.35, 95% CI [3.93, 17.74], p<0.001) and at one year (34.6% vs. 4.0%, HR 8.91, 95% CI [5.70, 13.94], p<0.001). NE occurring within 24 hours of the index treatment had similar impact on one-year mortality compared to NE occurring >24 hours after the index treatment (34.4% vs. 35.9%, HR 0.55 [0.16, 1.87], p=0.33). Non-CABG-related major bleeding events at 30 days were greater among patients with NE compared to those without NE (Table 5). In a time-adjusted multivariable analysis, the occurrence of NE was a strong independent predictor of all-cause mortality at 30 days (HR 4.07, 95% CI [1.49, 11.11], p<0.001) and one year (HR 4.25, 95% CI [2.37, 7.62], p<0.001), and of MACE (HR 2.27, 95% CI [1.29, 4.02], p=0.004) at one year. When the impact of stroke versus TIA on one-year all-cause mortality was separately considered, mortality was significantly increased after the occurrence of stroke (HR 4.16, 95% CI [2.13, 8.13], p<0.001) but not after TIA (HR 1.52, 95% CI [0.21, 10.83], p=0.68).
Discussion
The present analysis is the first to examine the short- and long-term rates and impact of NE in patients with moderate- and high-risk NSTEACS treated with an early invasive strategy using contemporary therapies. The major results of this study are as follows: 1) approximately 0.5% of patients with NSTEACS developed an NE within one year, with stroke comprising 81% of these events; 2) NE were significantly more common in patients treated with CABG compared to PCI or MT; 3) the occurrence of NE was strongly associated with increased risk of all-cause mortality and MACE at one year; 4) stroke, but not TIA, was a powerful independent predictor of subsequent mortality in NSTEACS.
Prior studies have examined the incidence and correlates of stroke in NSTEACS and after revascularisation procedures. Cronin and colleagues reported a six-month stroke risk of 1.3% among >18,000 patients with NSTEACS undergoing PCI or CABG5. CABG was the strongest independent predictor of stroke (HR 4.60, 95% CI [3.39, 6.34], p<0.0001), and those who experienced stroke had a fourfold increase in six-month mortality (27.0% vs. 6.3%, p<0.001). Of all strokes, 21% and 53% occurred within the first seven and 35 days of initial hospitalisation, respectively. In this study the rate of stroke with MT was not specified, and nearly all patients were treated with heparin, whereas in our study enoxaparin use and bivalirudin use were associated with higher and lower rates of NE, respectively. A study of 706,782 unselected patients from the National Cardiovascular Data Registry (NCDR) reported an incidence of periprocedural stroke (from cardiac catheterisation until hospital discharge) of 0.22% after PCI6. Of note, >80% of patients experiencing a stroke in the NCDR report presented with either NSTEACS or STEMI. Initial presentation with NSTEACS (OR 1.58, 95% CI [1.37, 1.82], p<0.0001) and STEMI (OR 3.23, 95% CI [2.71, 3.86], p<0.0001) were the two most powerful predictors of stroke after PCI. Again, outcomes with MT were not reported. Finally, a recent meta-analysis including 10,944 patients from 19 randomised trials of PCI vs. CABG found that CABG was associated with significantly higher rates of stroke at 30 days (1.20% vs. 0.34%, OR 2.94, 95% CI [1.69, 5.09], p<0.0001)7. The present study, in which the rates of stroke after MT, PCI, and CABG were tracked through one year, confirms and extends the results of these prior investigations. We observed that the one-year NE rate was substantially higher after CABG than after PCI (1.1% vs. 0.3%, respectively), and that NE was not less common with MT than with PCI (0.6% vs. 0.3%, respectively).
The reported rates of stroke in the present and prior reports have varied. In the SYNTAX trial, the one-year rate of stroke was significantly higher with CABG compared to PCI (2.2% vs. 0.6%, p=0.003), whereas this difference was not statistically significant in the FREEDOM trial (1.9% vs. 0.9%, p=0.06)1,7,10. In contrast, the one-year rates of stroke after both CABG and PCI in the present study were significant and substantially lower than those of SYNTAX and FREEDOM (0.85% and 0.28%, respectively, p=0.004). Stroke was a major component of the primary endpoint in SYNTAX and FREEDOM, but not in ACUITY, and thus ascertainment bias differences cannot be ruled out. However, the rate of NE may also be associated with the extent and complexity of coronary artery disease. Recent data for four-year stroke risk in SYNTAX suggest that the SYNTAX score is correlated with stroke risk in PCI, with higher scores conferring higher stroke risk3. In this regard, the mean SYNTAX scores of the PCI cohort in the SYNTAX and FREEDOM trials were 28.4 and 26.2, respectively, compared to 11.5 in the ACUITY trial1,7,11. Moreover, in the PCI treatment arm of our study, the presence of thrombus, moderate to severe calcification, and complexity of the treated lesions were all associated with NE. An association between coronary atherosclerosis and propensity to NE may be explained by: 1) additional catheter manipulations required in complex coronary disease with subsequent embolisation of debris; 2) coronary atherosclerosis, calcifications, and associated comorbidities (i.e., diabetes) serving as markers of extra-cardiac atherosclerotic disease (aorta, carotid, intracerebral); and/or 3) coronary thrombus reflecting systemic inflammation with multiple concurrent (intracoronary and extracoronary) plaque ruptures12. This indeed may represent the cause of higher rates of NE in CABG: that is, greater coronary atherosclerotic burden is more likely to be treated with CABG, and is therefore associated with a greater risk of NE. However, a number of prior studies examining PCI and CABG individually have failed to show correlations of stroke risk with number of vessels involved, number of arteries bypassed, number of stents placed or the characteristics of the lesions8,9. Nonetheless, vessel characteristics have not been directly compared in PCI and CABG populations with active NSTEACS and subsequent NE.
The finding of a relatively high rate of NE in the MT arm (especially when compared to the PCI group) is noteworthy. NSTEACS patients who receive MT after being considered for early invasive treatment are a heterogeneous group composed of those with 1) severe and extensive CAD not suitable for either PCI or CABG, and 2) less severe (or absent) CAD. In this case, diffuse/small vessel coronary disease and/or the presence of multiple comorbidities (i.e., porcelain aorta) may be associated with a higher risk of NE after diagnostic angiography.
We observed that most NE occurred early after invasive procedures (angiography, PCI, or CABG), which is consistent with other prior studies1,3,5. This finding suggests possible “iatrogenic” procedure-related neurologic complications. However, not all NE occurred on the day of the procedure, and increased susceptibility to NE may also be present in NSTEACS due to a systemic inflammatory or hypercoagulable state. In this regard, the presence of elevated biomarkers of inflammation in NSTEACS has been previously associated with a higher risk of ischaemic and bleeding events and a less favourable prognosis13,14. The association of higher baseline white blood cell count and C-reactive protein level with subsequent NE in our study is concordant with these earlier reports. A “delayed” invasive approach for NSTEACS patients, allowing a “cooling down” period to abate systemic inflammation, might result in a lower risk of NE. However, heightened systemic inflammation can last for months after presentation with ACS, and prior studies have not suggested improved ischaemic outcomes with a waiting period in NSTEACS15,16.
Interestingly, rates of NE in patients receiving enoxaparin were higher than in those receiving heparin or bivalirudin. NE rates were 0.40% in patients receiving bivalirudin, 0.43% in patients receiving heparin, and 0.96% in patients receiving enoxaparin. Prior studies comparing a glycoprotein IIb/IIIa inhibitor in combination with either enoxaparin or heparin have shown no differences in TIA or stroke (both haemorrhagic and ischaemic) when specifically examined (as opposed to TIMI major and minor haemorrhage criteria)17-19. Further, to our knowledge, few studies have looked at NE in the setting of bivalirudin use, this being one of them.
We note that CHADS2 and CHA2DS2-VASc scores were associated with NE in our NSTEACS population. Although not surprising given that these scores represent well-known risk factors for atherosclerotic disease, this observation suggests that CHADS2 or CHA2DS2-VASc scores may predict not only the risk of embolic stroke associated with atrial fibrillation but also the risk of NE after revascularisation and in patients treated with MT. A recent study suggested that these scoring systems may have a greater predictive value for NE in a non-atrial fibrillation population with coronary disease than in an atrial fibrillation population20. While this is interesting, further prospective validation is required.
Notably, all 17 patients developing NE after surgery underwent on-pump CABG, whereas no events were observed after off-pump surgery. While the benefit of off-pump compared to on-pump CABG surgery in preventing stroke is still a matter of debate21-25, the results of the present study reinforce the need for approaches to prevent NE after surgery, especially in those undergoing on-pump CABG.
Limitations
Several limitations of the present report should be discussed. The current study represents a post hoc examination of NE from the ACUITY trial, and NE events were not centrally adjudicated, which may have introduced bias. Assessments from neurologists and the results of imaging tests were not collected. Therefore, no distinction was drawn between ischaemic and haemorrhagic NE. Further, while an association has been shown between the presence of thrombus, moderate to severe calcification, and complexity of treated lesions, only 50% underwent quantitative coronary angiography, thereby potentially overestimating or underestimating this association. NE may have been under-reported, especially minor events in the early postoperative period after CABG, when intubation and sedation may preclude adequate neurologic examination. Finally, the occurrence of NE events beyond one year was not assessed.
Conclusion
These limitations notwithstanding, in patients with moderate- and high-risk NSTEACS undergoing an early invasive strategy in the large-scale ACUITY trial, CABG was associated with a higher risk of NE at 30 days and one year compared to PCI or MT alone. The increased rate of events with CABG principally occurred within 30 days of surgery, and was otherwise similar among the three treatments for the remainder of one year. Despite its limited incidence, the occurrence of NE in patients with NSTEACS was associated with high mortality, both early and late.
| Impact on daily practice From the 13,819 patients with moderate- and high-risk NSTEACS enrolled in the large randomised ACUITY trial, neurological events (NE) at one year were significantly higher in patients treated by CABG (1.1%) compared to PCI (0.3%) or medical therapy (0.6%), p<0.001. Independent predictors of NE at 30 days and one year included age, renal insufficiency, baseline troponin elevation, and initial treatment with CABG. The occurrence of NE was a strong independent predictor of death at 30 days and one year (HR 4.07, 95% CI [1.49, 11.11], p=0.006, and HR 4.25, 95% CI [2.37, 7.62], p<0.001, respectively). These findings may have an important impact when choosing the optimal revascularisation strategy for patients presenting with NSTEACS. |
Conflict of interest statement
R. Mehran has received research grants from Sanofi-Aventis, The Medicines Company, Abbott Vascular, Bristol-Myers Squibb, and AstraZeneca, and has served as consultant/advisory board member for Eli Lilly and Daiichi Sankyo. G.W. Stone has served as consultant to Abbott Vascular, Boston Scientific, Medtronic, The Medicines Company, Eli Lilly, Daiichi Sankyo, and Merck. The other authors have no conflicts of interest to declare.

