
Medical management with a view to invasive therapy in adults with non-ST-elevation myocardial infarction (NSTEMI)1 is the recommended standard of care. However, older patients are significantly less likely to receive pharmacological therapies and undergo invasive management, such as angiography and percutaneous coronary intervention (PCI), compared to younger patients. This may partly explain why older people are more likely to have adverse outcomes following acute coronary syndrome (ACS), with a 15.7x increased odds of in-hospital mortality in patients ≥85 years old compared to those <45 years2. The global population is ageing and consequently the number of older patients presenting with ACS continues to increase. Despite the rising numbers of older patients presenting with ACS and their documented poorer clinical outcomes, there is currently a lack of sufficient studies to guide management in this specific population.
In this issue of EuroIntervention, the study by Llaó et al3 adds some much needed data on the management of older patients with ACS, focusing specifically on non-ST-elevation acute coronary syndromes (NSTEACS).
This prospective observational study examines the effect of an invasive strategy in older patients with NSTEACS, and the impact of frailty on six-month outcomes compared to routine medical management. The main finding was that the older patients who received medical treatment alone (i.e., no invasive intervention) had worse outcomes at six months. Interestingly, however, the beneficial effects of invasive management were only seen in non-frail patients. The authors therefore imply that invasive management is optimal in older NSTEACS patients, but that patient frailty may reduce its benefits. However, the nature of the study design with the relatively low sample size makes the findings interesting but not conclusive.
As this study is observational by design, there are inherent biases which make the results questionable. The mean age of 83.4±4 years is an appropriate average age for a study of older patients. The patients in the conservative treatment group were on average three years older than patients in the invasive treatment group. Although the difference in age appears relatively small between the two groups, it is significant in older patients, creating bias towards reduced adverse outcomes in the invasive treatment group. When assessing baseline frailty, the 5-point FRAIL scale score was used. This rapid assessment tool has been validated against other frailty scores routinely used in older individuals4. It is, however, questionnaire-based; a score incorporating objective frailty measures, such as the FRIED frailty score which measures hand grip strength and gait speed, may have been a more accurate assessment of patient frailty. Nevertheless, the study classified 27.3% of its cohort as frail, echoing findings from a large systematic review of frailty amongst older individuals which concluded that 15.7% of patients aged 80-84 years and 26.1% of patients 85+ years were frail5.
The study population of Llaó et al comprised 531 patients aged ≥80 years from 44 sites. This fairly small sample size, whilst being sufficiently powered for primary outcome analysis comparing the invasive and conservative management groups based on the study design paper’s power calculation, leaves the frailty subgroup analysis underpowered and hence these results cannot be deemed conclusive6. In addition, multivariate analysis in these subgroups creates high potential for optimism, overfitting and miscalibration, hence the findings may not be representative of the general population. The results need to be confirmed with internal and/or external validation and, as acknowledged by the authors, repeated in a larger randomised controlled trial (RCT) of frail NSTEACS patients to confirm whether invasive management is optimal in this selective but ever-growing older patient population.
Whilst this was a fairly small cohort, the ability to recruit enough older patients to reach the target sample size in this study should be acknowledged, given the notorious difficulty in recruiting this patient population into cardiovascular clinical trials. Indeed, the three RCTs mentioned in the study, namely the After Eighty, MOSCA and Italian Elderly ACS trials, all struggled to reach their recruitment targets, subsequently resulting in either under-recruitment with consequential underpowered statistical analysis, or, in the case of the After Eighty study, extension of the recruitment period to reach the full recruitment target (Table 1)7-9.
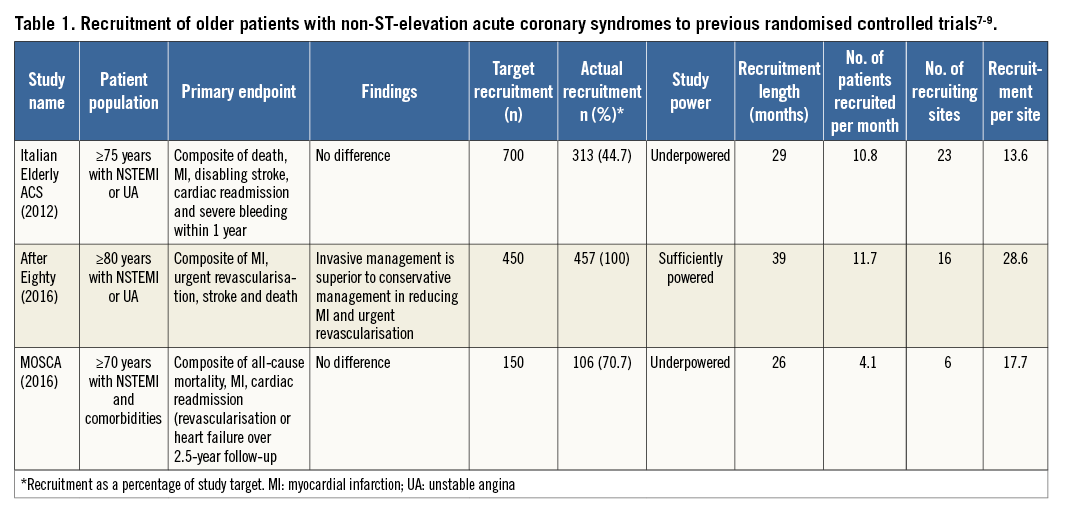
The study by Llaó et al acknowledged the efforts of the few previous RCTs with a total sample size of 786 patients addressing this research question; however, interestingly, these either remained inconclusive or their results contradicted one another (Table 1)7-9. Excitingly, the ongoing British Heart Foundation’s older patients with non-ST SEgmeNt elevatIOn myocaRdial infarction Randomized Interventional TreAtment (BHF SENIOR-RITA, NCT03052036) trial strives to provide conclusive answers to this pertinent research question. This UK-wide multicentre RCT aims to recruit 2,300 NSTEMI patients aged ≥75 years, randomising them to either optimal medical therapy (OMT) alone or OMT with invasive management, with a view to revealing which management strategy is of most benefit to older patients presenting with NSTEMI. Whilst its primary outcomes focus on one-year cardiovascular death or non-fatal myocardial infarction, the study also includes assessments of frailty, cognition, comorbidity and quality of life as secondary outcome measures.
Overall, the study by Llaó et al provides interesting insight into the optimal management of NSTEACS in older patients and the possible influence of patient frailty on intervention outcomes. However, given the observational nature of this paper and the associated inherent risk of bias, the researchers rightly concluded that the study’s results serve as a hypothesis generator rather than clear evidence for better six-month outcomes with invasive management in older patients. Larger randomised controlled trials such as the ongoing BHF SENIOR-RITA trial should provide definitive evidence on how best to manage older NSTEACS patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

