Abstract
Background: Historically the elderly have been under-represented in non-ST-elevation myocardial infarction (NSTEMI) management trials.
Aims: The aim of this trial was to demonstrate that an intervention-guided strategy is superior to optimal medical therapy (OMT) alone for treating NSTEMI in elderly individuals.
Methods: Patients (≥80 years, chest pain, ischaemic ECG, and elevated troponin) were randomised 1:1 to an intervention-guided strategy plus OMT versus OMT alone. The primary endpoint was a composite of all-cause mortality and non-fatal myocardial reinfarction at 1 year. Ethics approval was obtained by the institutional review board of every recruiting centre.
Results: From May 2014 to September 2018, 251 patients (n=125 invasive vs n=126 conservative) were enrolled. Almost 50% of participants were female. The trial was terminated prematurely due to slow recruitment. A Kaplan-Meier estimate of event-free survival revealed no difference in the primary endpoint at 1 year (invasive 18.5% [23/124] vs conservative 22.2% [28/126]; p=0.39). No significant difference persisted after Cox proportional hazards regression analysis (hazard ratio 0.79, 95% confidence interval 0.45-1.35; p=0.39). There was greater freedom from angina at 3 months (p<0.001) after early intervention but this was similar at 1 year. Both non-fatal reinfarction (invasive 9.7% [12/124] vs conservative 14.3% [18/126]; p=0.22) and unplanned revascularisation (invasive 1.6% [2/124] vs conservative 6.4% [8/126]; p=0.10) occurred more frequently in the OMT alone cohort.
Conclusions: An intervention-guided strategy was not superior to OMT alone to treat very elderly NSTEMI patients. The trial was underpowered to demonstrate this definitively. Early intervention resulted in fewer cases of reinfarction and unplanned revascularisation but did not improve survival.
Introduction
The very elderly (≥80 years old) are typically under-represented in randomised trials of non-ST-elevation myocardial infarction (NSTEMI) management. Indeed, a systematic review of 1,067,520 patients from 460 acute coronary syndrome (ACS) trials conducted between 2001 and 2018 found the mean (standard deviation [SD]) age of enrolled individuals to be just 62.9 (10.7) years overall1. Furthermore, the evidence base used to support contemporary practice guidelines in the elderly comprises mainly observational data2,3,4,5,6,7,8 and meta-analyses9,10. Randomised controlled trial data are scant - heterogeneous in relation to what age stratum is designated elderly and beset by small sample populations11,12. Indeed, at the time of recruitment to the RINCAL trial, only the Italian Elderly ACS trial specifically investigating an optimal strategy for NSTEMI in the elderly (≥75 years) had been published. Yet, despite the paucity of definitive data, European guidelines published in 2016 maintained their support for an early intervention-guided strategy for elderly individuals13. There remains, however, a widespread reluctance to offer revascularisation to elderly patients based on frailty, reduced life expectancy, impaired cognition leading to a presumed attrition in pharmacotherapy compliance, polypharmacy, and a greater susceptibility to drug- and procedure-related complications such as renal failure and bleeding3.
We therefore performed a randomised trial of adequate statistical power and follow-up to prove the hypothesis that an intervention-guided strategy is superior to an initially conservative management approach in very elderly NSTEMI patients with respect to a combined endpoint of all-cause mortality or repeat non-fatal myocardial reinfarction at one year. Our aim was to ensure that this cohort should receive evidence-based care irrespective of age.
Methods
TRIAL DESIGN
The Revascularisation or medIcal therapy iN elderly patients with aCute anginAL syndromes (RINCAL) trial was a pragmatic investigator-initiated multicentre open-label randomised controlled trial of an intervention-guided strategy supplemented with optimal medical therapy (OMT) versus OMT alone in NSTEMI patients aged ≥80 years. An NSTEMI was defined as an acute hospital admission characterised by chest pain associated with typical ischaemic changes on electrocardiogram (ECG) and a significant rise in cardiac troponin (T or I) relative to the specific parameters of the assay used. Eligibility was predicated on the enrolling cardiologist confirming that the patient was suitable both for an intervention-guided or for an initially conservative strategy. Exclusion criteria included acute ST-elevation myocardial infarction (MI), cardiogenic shock, platelet count ≤50 × 109/mm3, life expectancy <1 year, allergies to antiplatelet therapy, major gastrointestinal haemorrhage within the preceding three months or any previous intracranial haemorrhage. The full trial protocol is shown in Supplementary Appendix 1.
Eligible patients were treated according to the established institutional care pathway, inclusive of loading doses of aspirin and a P2Y12 inhibitor (if antiplatelet naïve) and a therapeutic dose of low molecular weight heparin or factor Xa inhibitor. Patients were approached at this point and gave informed consent if they were agreeable to trial participation. Randomisation was then performed 1:1 via a web-based system (https://www.e-dendrite.com).
The trial was approved by the National Research Ethics Service Committee South East Coast: Brighton and Sussex and the institutional review boards of each participating interventional centre. The trial was performed according to the Declaration of Helsinki and Good Clinical Practice with an unrestricted educational grant from Medtronic (Dublin, Ireland). The funder had no role in study design, data analysis or drafting of the manuscript. The trial was prospectively registered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02086019)
PROCEDURES
For individuals randomised to an invasive strategy, coronary angiography was conducted with physiological determination of intermediate lesions by fractional flow reserve or instantaneous wave-free ratio at the operator’s discretion. Significant coronary lesions were treated with ad hoc percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) surgery or referred directly for further discussion by a Heart Team. Use of second-generation drug-eluting stents for PCI and arterial conduits for CABG surgery was strongly recommended. Coronary anatomy not requiring or amenable to intervention was subsequently managed with OMT. Troponin was measured on admission (in all patients), immediately prior to invasive testing and again 16-22 hours following revascularisation, or as near to discharge if sooner.
OMT alone patients were permitted to have diagnostic angiography if there was ongoing chest pain with or without dynamic ECG changes and/or further rise in troponin levels.
At discharge, all patients were to be prescribed dual antiplatelet therapy for at least six months. Aspirin was mandatory (unless contraindicated) with the choice of P2Y12 agent left to operator discretion. Rate control medication (beta-blockers, calcium channel blockers, or ivabradine), an angiotensin-converting enzyme inhibitor (or equivalent), high dose statins, and coronary vasodilator drugs (as required) were encouraged. Proton pump inhibitors were mandated for a prior history of indigestion or gastrointestinal bleed. Follow-up at 3, 6, and 12 months recorded well-being, drug compliance and adverse clinical events.
ENDPOINTS
The primary composite endpoint was all-cause mortality and non-fatal myocardial reinfarction at one year post randomisation. Reinfarction was defined as a new episode of cardiac chest pain associated with a rise in troponin exceeding the 99th percentile of a normal population (i.e., upper limit of normal [ULN] for the assay used). Given that the trial inclusion criteria stipulated a significantly raised troponin on admission, periprocedural MI was defined as a rise in troponin >20% of the baseline value when it was above the 99th percentile ULN, but stable or falling, as per the third universal definition of MI14.
Major secondary endpoints were time to death or non-fatal reinfarction, unplanned revascularisation, permanent stroke (i.e., new neurological deficit with duration >24 hours confirmed by a neurologist and appropriate neuro-imaging), major bleeding (Bleeding Academic Research Consortium type 3B or above) during hospital admission and at one year, deterioration in renal function during hospital admission, angina burden at three months and one year and stent thrombosis at one year.
STATISTICAL ANALYSIS
The rate of the primary endpoint was estimated to be 38% after conservative management and 28% in the invasive cohort at one year11,12. Therefore, 750 patients (allowing for a 5% loss to follow-up) were targeted for randomisation to achieve 80% power (two-sided α=0.05) to detect a 10% difference in the means.
Continuous variables are summarised as mean±SD, or as median and interquartile range (IQR) where appropriate. Unadjusted differences were assessed with the two-sample t-test, or two-sample Wilcoxon rank-sum test. Categorical variables are presented as absolute numbers and percentages and compared using the χ2 or Fisher’s exact test. Event-free survival was estimated using the Kaplan-Meier method and differences assessed using the log-rank test. Survival was examined as a time-to-event outcome. A Cox proportional hazards regression model was applied for multivariable adjustment. A value of p<0.05 was used for statistical significance. Analysis was conducted on an intention-to-treat basis and was performed using R version 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Data were stored on a dedicated web-based secure site, collated by the host institution, and validated by the trial organisation (Sussex Cardiac Centre, Brighton, UK). All clinical events were adjudicated by an independent committee. The Data and Safety Monitoring Committee (DSMC) was responsible for interim data analysis.
Results
From May 2014 to September 2018, 12 interventional centres in the UK recruited 251 NSTEMI patients who were subsequently randomised to an intervention-guided (n=125) or OMT alone (n=126) strategy. One patient withdrew from the trial after giving consent (Figure 1). The DSMC recommended cessation of the trial before the sample target was met due to slow recruitment. Trial visits ended in September 2019 with all patients recruited completing at least one-year follow-up.
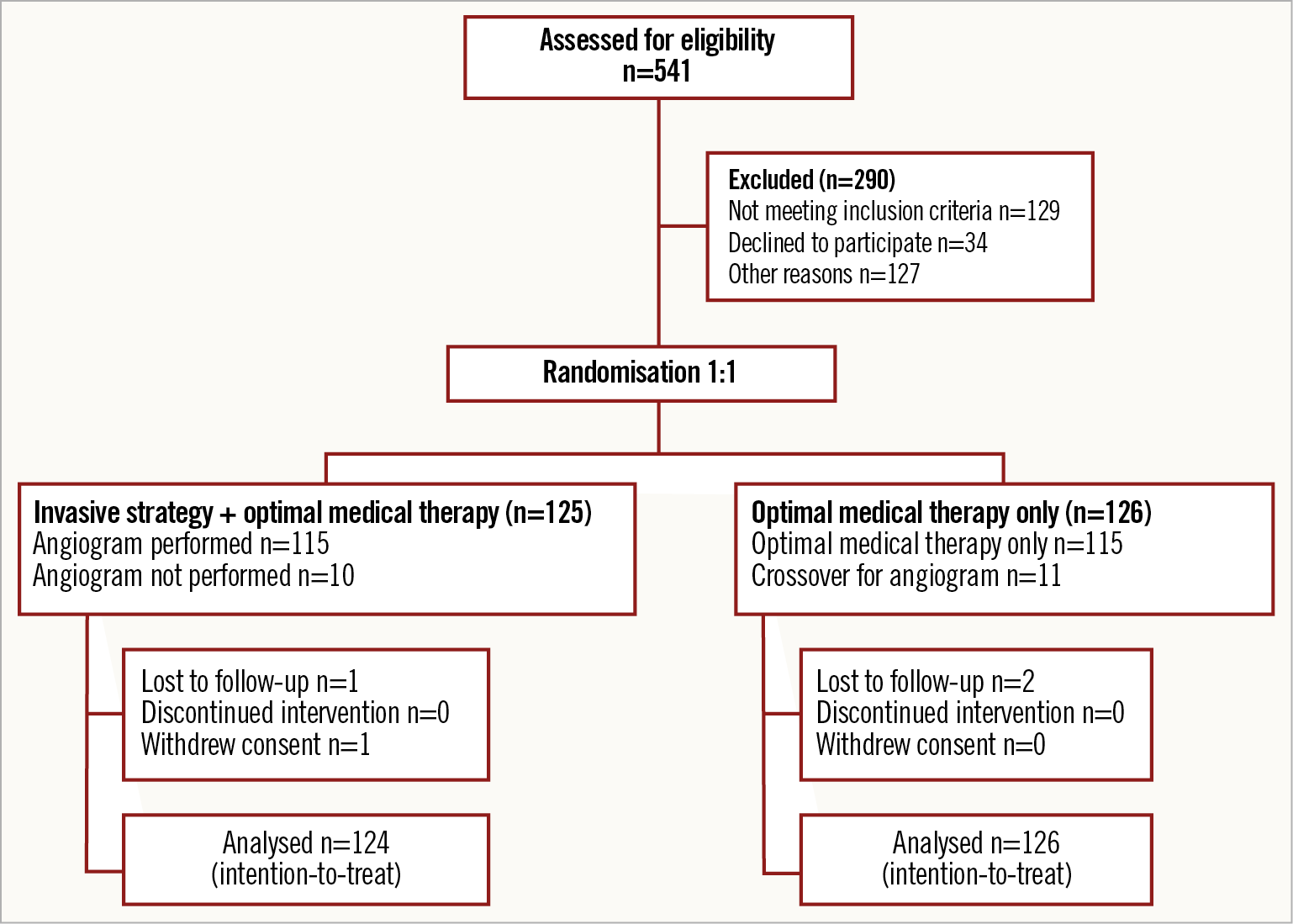
Figure 1. The RINCAL trial CONSORT diagram.
BASELINE DEMOGRAPHICS AND PROCEDURES
The trial arms were well matched for baseline demographics and cardiovascular risk (Table 1). In the invasive arm, nine patients (7.3%) did not proceed to an angiogram. Coronary angiography was predominantly performed using radial access (83.3%). The mean time (±SD) from randomisation to angiography was 2 (±2) days in the invasive treatment arm. Over 50% of those randomised to intervention needed revascularisation either by PCI or by CABG. Eleven patients (8.7%) in the conservative arm subsequently required an angiogram for clinical instability. Of these, nine patients (81.8%) had two-vessel coronary artery disease (CAD) or greater. Four patients (44.4%) received revascularisation. Uptake of guideline-mandated drug therapies was high. Nitrates and nicorandil were prescribed more often in the OMT alone cohort (Table 2). One-year follow-up was achieved for 98.8% of the trial participants who completed the in-hospital protocol. The median duration of follow-up was 369 days (IQR 354-380 days). All those patients completing the trial protocol received at least six months of dual antiplatelet therapy.
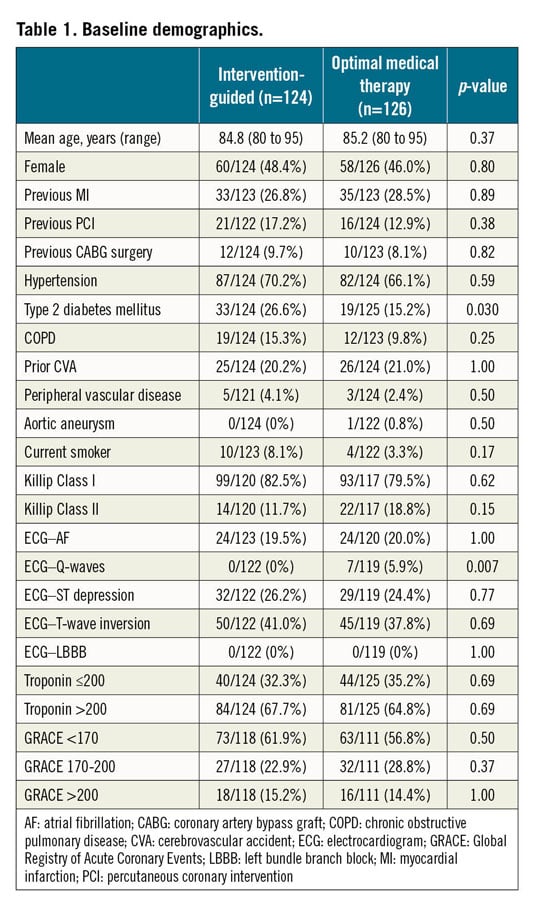
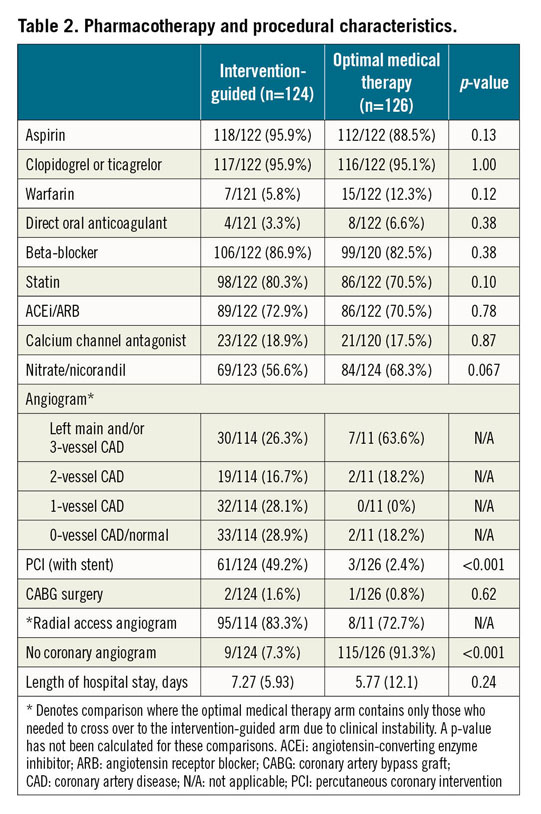
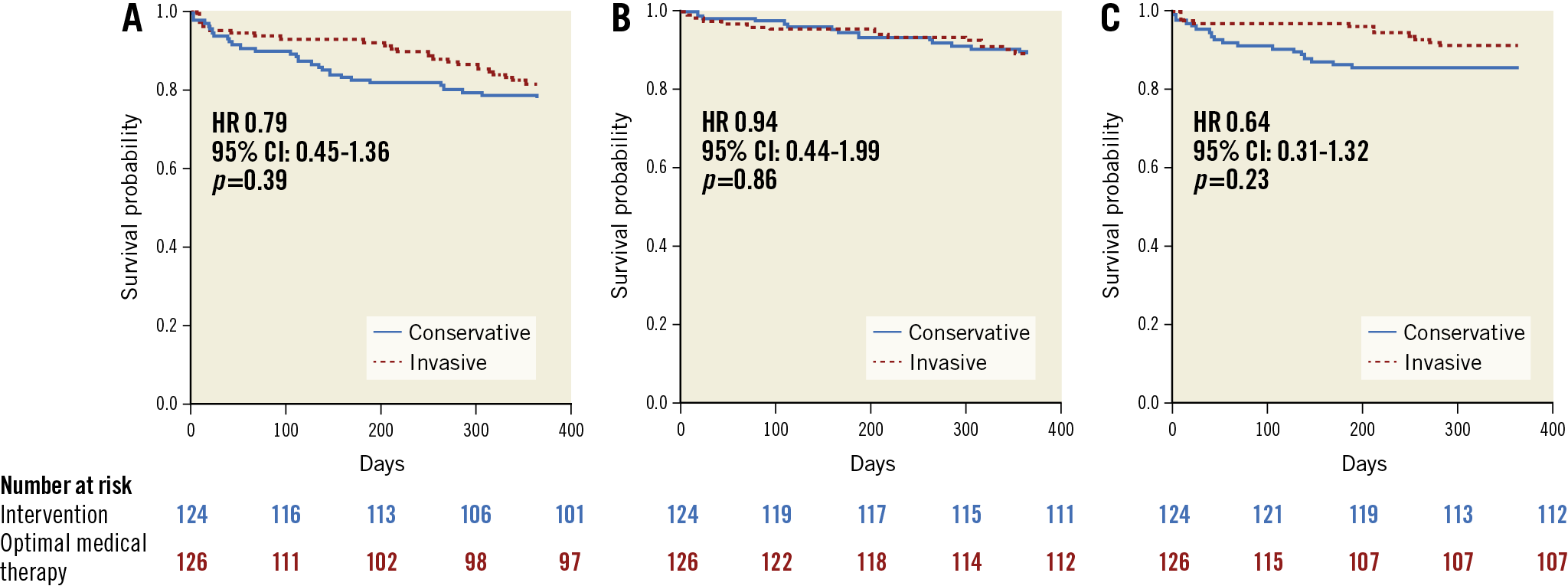
Figure 2. Kaplan-Meier survival analyses. A) Primary combined endpoint. B) Time to all-cause death. C) Time to non-fatal myocardial reinfarction. CI: confidence interval; HR: hazard ratio
PRIMARY COMBINED ENDPOINT
There was no significant difference in the combined primary endpoint based on a Kaplan-Meier estimate of event-free survival (invasive 18.5% [23/124] vs conservative 22.2% [28/126]; p=0.39) (Figure 2A). A Cox proportional hazards regression analysis confirmed the equipoise (hazard ratio [HR] 0.79, 95% confidence interval [CI]: 0.45-1.35; p=0.39). There were no significant differences observed in the individual components of the primary endpoint (Figure 2B, Figure 2C, Table 3).
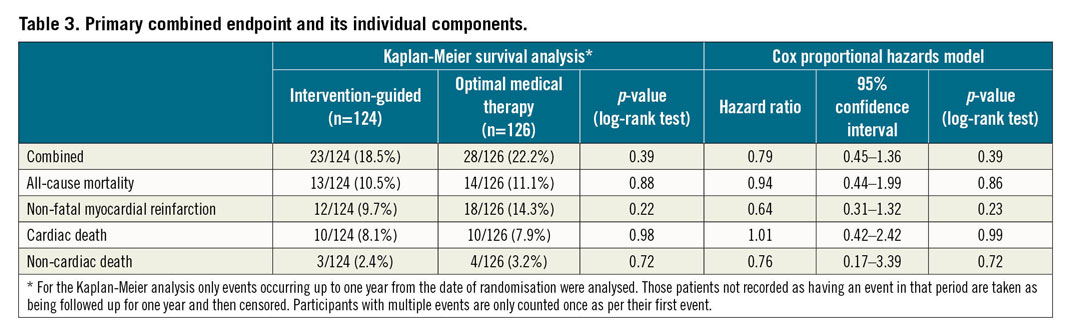
Multivariable analysis using a Cox proportional hazards model found no significant interaction with the primary endpoint after adjustment for important variables (Figure 3).
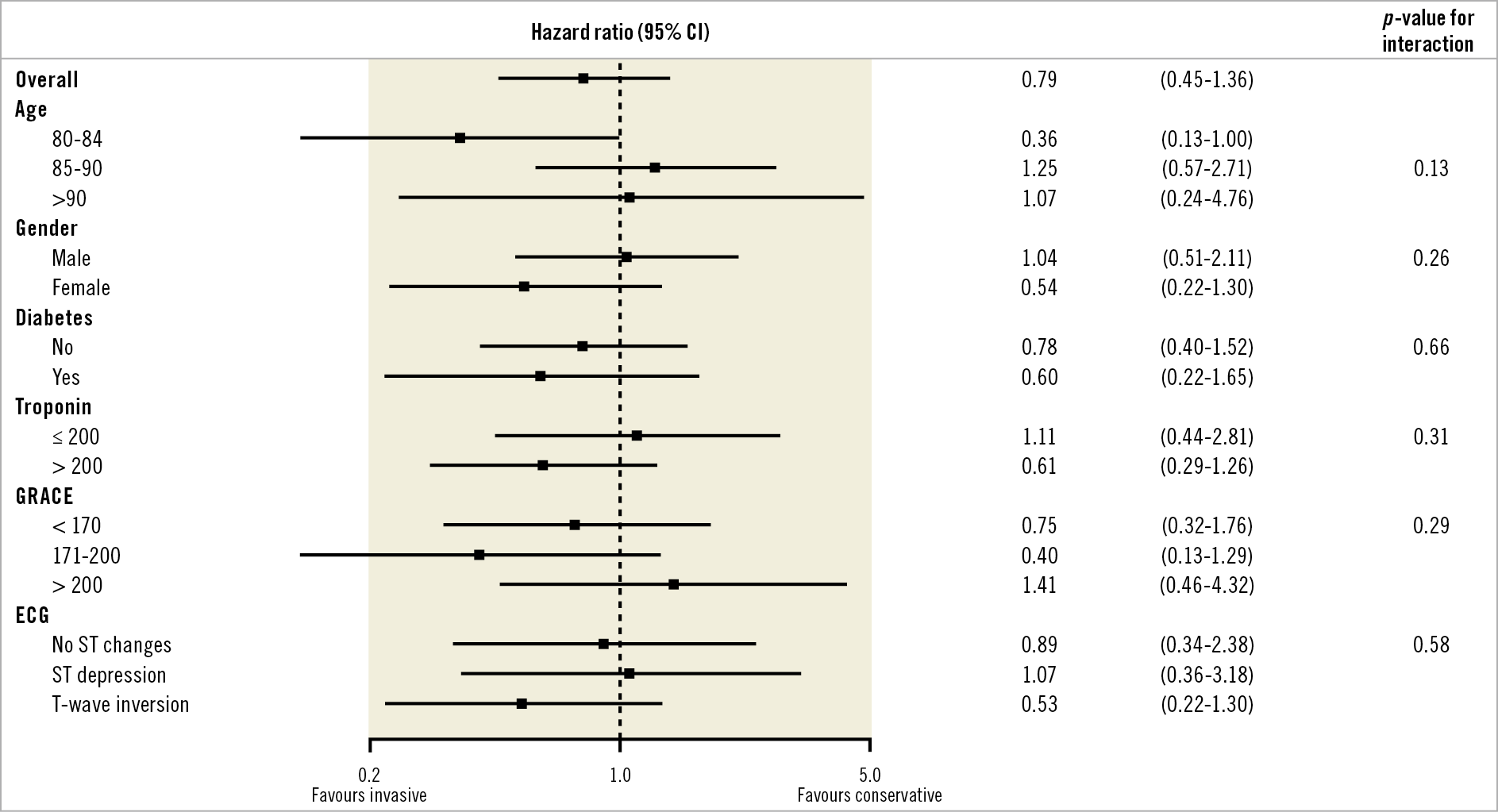
Figure 3. Multivariable Cox proportional hazards analysis. The primary combined endpoint adjusted for important subgroups. CI: confidence interval; ECG: electrocardiogram; GRACE: Global Registry of Acute Coronary Events
SECONDARY ENDPOINTS
These were largely similar in both trial arms (Table 4). Angina burden was significantly less marked after an intervention-guided strategy for the first three months but this too reached equipoise by one year.
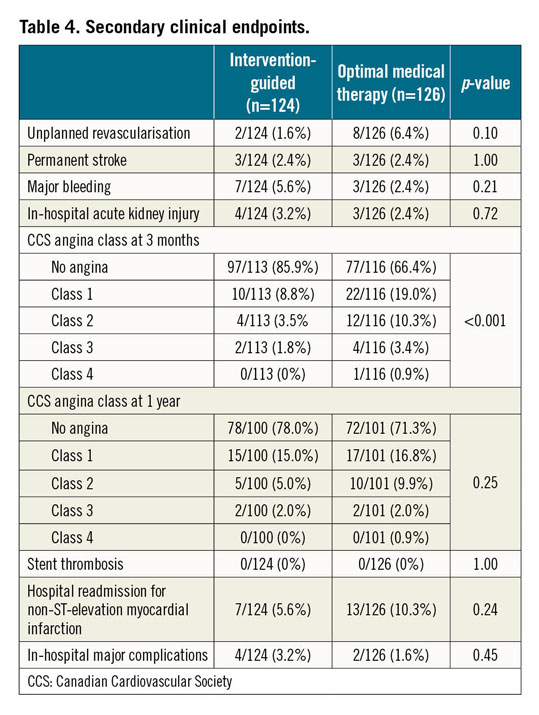
When unplanned revascularisation was incorporated with the primary combined endpoint of all-cause mortality and non-fatal reinfarction at one year, there remained no significant difference between the strategies after a Kaplan-Meier survival analysis (invasive 18.5% [23/124] vs 24.6% [31/126]; p=0.19). A Cox proportional hazards model also showed no difference (HR 0.70 [0.41-1.20]; p=0.19).
Discussion
The RINCAL trial demonstrated clinical equipoise at one year when an intervention-guided strategy with OMT was compared to OMT alone in treating intermediate- to high-risk very elderly NSTEMI patients. Almost half of the participants were female, which corresponds favourably when compared to the number found by a recent systematic review in which the overall female proportion constituted just 32.0% of previous European ACS trials1.
There were no significant subgroup differences although there was a trend towards favouring an invasive strategy in those aged 80-84 years whereas those ≥85 years appeared to do better with OMT alone. An invasive strategy substantially improved angina burden compared to conservative management at three months. Angina burden was equivalent by one year. Importantly, this was driven by enhanced symptom control in the OMT alone patients rather than a deterioration in the interventional arm. No stent thrombosis occurred in those proceeding to PCI despite a notable level of multivessel CAD and a strong likelihood of variable drug compliance.
Recruitment to the trial was difficult and led to the DSMC recommending early cessation of the study on the grounds of futility in reaching the target sample size. Contrast this to the XIMA trial, where we previously compared drug-eluting stents versus bare metal stents in octogenarian patients presenting with stable angina or NSTEMI15. Despite a similar age group and underlying pathology, recruitment was straightforward and timely. The question posed by XIMA, however, was not whether to revascularise but rather how to revascularise.
There are likely to be several reasons for slow recruitment. Of the 20 interventional centres signed up to the trial, only 12 sites were able to recruit patients over a five-year period. During that time, only 541 patients were assessed for eligibility according to the centre-specific screening logs, with over half eventually excluded.
The eligibility criteria effectively required patients haemodynamically stable enough for randomisation to an initially conservative strategy. We know that the very elderly present unique challenges in this context. They are more likely to have multiple comorbidities, varying degrees of frailty, polypharmacy and extensive multivessel CAD, which alone or in combination can exacerbate the risk of mortality, recurrent/ongoing myocardial ischaemia, and heart failure4,7,9. Moreover, advanced age alone is an independent predictor of adverse outcome after ACS3,13. Thus, despite the absence of clinical instability, such unfavourable characteristics may have deterred investigators from recruiting more patients because they felt either that a routine invasive strategy was guideline-mandated or that the risk of complications associated with intervention far outweighed any benefits – a risk-treatment paradox. Anecdotally, several trial recruiters later intimated that patient preference was also one of the most common reasons for exclusion from the trial.
Alternatively, the screening process could be viewed as a pragmatic real-world reflection of how experienced interventional cardiologists assess their very elderly NSTEMI patients. Only one patient withdrew from the trial; the remainder completed the in-hospital protocol. Crossover from the conservative to the invasive arm of the trial was low, and wholly appropriate when clinical instability had developed. The rates of unplanned revascularisation, stroke, acute kidney injury and rehospitalisation for reinfarction were modest overall and equitable between the trial arms. This is in spite of the majority of trial participants being strongly biomarker positive with ischaemic ECG changes and mostly intermediate- to high-risk for in-hospital and six-month mortality according to their Global Registry of Acute Coronary Events score (Table 1). Major bleeding was also relatively uncommon despite almost blanket administration of dual antiplatelet therapy in what was a high bleeding risk population. The predominant use of radial access angiography and glycoprotein inhibitors being discouraged are likely to have contributed to maintaining major bleeding at an “acceptable” level.
The RINCAL trial is at odds with previous observational and randomised data. Retrospective analysis of the German ACOS, Australian ACACIA, Spanish LONGEVO-SCA and GRACE registries and post hoc analysis of the TACTICS-TIMI 18 trial all demonstrated net clinical benefit from an intervention-guided strategy in terms of in-hospital, six-month and one-year outcomes in those aged ≥75 years3,4,5,6,8. They also confirmed that the elderly, despite being at greatest risk (yet more likely to benefit from intervention), were less likely to be prescribed guideline-mandated pharmacotherapy, be offered early invasive imaging or receive revascularisation3,4,7. Much of these data, however, are at least 10 to 20 years old.
There have been just two other randomised trials of optimal non-ST-elevation-ACS (NSTE-ACS) management conducted specifically in the elderly. The Italian Elderly ACS Study (patients ≥75 years with a mean age of 82) recruited 313 patients over a 29-month period (2008-2010) and demonstrated a 20% reduction in the composite primary endpoint of all-cause mortality, non-fatal MI, stroke and rehospitalisation for cardiovascular causes or bleeding. This was deemed non-significant given that the trial was powered for a 37.5% difference in the primary endpoint12. Of note, 29% of the conservative arm crossed over to an invasive strategy, mainly for recurrent ischaemia. The Norwegian After Eighty study (patients ≥80 years with a mean age of 85) enrolled 457 NSTE-ACS patients over a 38-month period (2010-2014)11. A wide primary combined endpoint of all-cause death, non-fatal MI, urgent revascularisation, and stroke helped to drive down the sample size target. Nevertheless, this trial accumulated sufficient power to demonstrate that an invasive strategy was superior to OMT alone with a significant 20.8% difference in the primary endpoint, driven primarily by a reduction in non-fatal MI and need for urgent revascularisation. Neither study demonstrated a mortality benefit for an intervention-guided strategy, in tandem with our findings. Importantly, recruitment to these trials predates the publication of the then European guidelines published in 2016, and indeed formed the basis of their subsequent recommendations13. The present investigation, however, recruited at a time when these guidelines were becoming increasingly entrenched in everyday practice, which may have adversely affected timely recruitment. The most recent European guidelines published in 2020 recommend the application of the same interventional strategies for older patients as used for younger patients, despite a continued lack of novel randomised or observational data16.
Limitations
The RINCAL trial was underpowered to test the original hypothesis adequately due to slow recruitment. It exemplifies the difficulty of recruiting to strategy-based investigations, especially when the baseline risk of the patient cohort is inherently high. Slow recruitment may have allowed protocol drift to develop. The adverse effects of trial drift are difficult to quantify, but primarily hamper timely recruitment rather than directly affect study endpoints. We did not collect quality-of-life measures, which may have provided more relatable outcome data for patients to appreciate.
The open-label nature of the trial design and the eligibility of very elderly NSTEMI patients left solely to the recruiting physician leave the trial susceptible to performance, detection and selection bias. Frailty and its effect on recruitment to the trial were not formally investigated or recorded in terms of a comorbidity index and/or functional assessment of the included and excluded; instead, the expert view of the cardiologist was key. To counter these putative weaknesses, we specifically chose high-volume interventional centres with experienced cardiologists to enrol participants. There was independent adjudication of clinical events. Also, the trial arms were well matched according to demographics, cardiovascular risk and pharmacotherapy. Moreover, the absence of a significant difference in all primary and secondary endpoints would go against overly intrusive biases confounding the final results, although it could be argued that these results ultimately reflect a lack of statistical power.
Conclusions
The RINCAL trial was terminated early due to challenging recruitment; however, at study termination there was no difference in the rate of one-year death and non-fatal myocardial reinfarction when an intervention-guided strategy (plus OMT) was compared to OMT alone in very elderly yet clinically stable NSTEMI patients deemed suitable for either management pathway. Due to its limited power, the trial was unable to show superiority of an intervention-guided strategy in this patient group. Moreover, the event rates were lower than expected, which suggests that medical therapy may be a reasonable treatment option for a proportion of this population. Contrariwise, an invasive approach appeared to be safe, resulted in numerically lower reinfarctions and reduced the need for unplanned revascularisation.
The trial accentuates the importance of individualised decision making to optimise outcome in very elderly NSTEMI patients, taking into consideration patient preference, life expectancy, cognitive and functional status, comorbidities, and inherent bleeding risk. A one size fits all approach should not be applied to this markedly heterogeneous patient group.
|
Impact on daily practice The optimal strategy for treating very elderly (≥80 years old) patients with intermediate- to high-risk NSTEMI remains poorly defined. The RINCAL randomised trial was conducted with the aim of showing that an intervention-guided strategy plus optimal medical therapy (OMT) was superior to OMT alone in octogenarians presenting with NSTEMI. At one year post randomisation there was no significant difference in the combined primary endpoint of all-cause mortality and non-fatal myocardial reinfarction after either strategy, but the trial was underpowered to confirm this definitively due to slow recruitment. |
Acknowledgements
We are indebted to Mrs Nicola Skipper, Mrs Ailie MacKenzie and Mr Duncan Fatz for their tireless efforts in the day-to-day management of the trial at the Sussex Cardiac Centre, Brighton, United Kingdom.
Funding
This work was supported by an unrestricted educational grant from Medtronic Clinical Research Institute (Dublin, Ireland).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

