
Background
The last three decades have borne witness to the “reperfusion revolution” in patients with ST-elevation myocardial infarction (STEMI). Widespread acceptance of the concept proposed by Reimer and Jennings in 1979 that “time is muscle” has provided the impetus for the establishment of networks and systems of care to ensure the provision of rapid reperfusion therapy to as many patients as possible.
At the same time, epidemiological trends in high-income countries have seen a decline in the incidence of STEMI and a corresponding increase in the numbers of patients with non-ST elevation myocardial infarction (N-STEMI), which now accounts for two thirds or more of all patients presenting with acute coronary syndrome (ACS). N-STEMI is characterised by pathophysiological and prognostic heterogeneity. It has always been assumed to be more benign than STEMI, but the data do not necessarily support this (Figure 1) and indeed up to 3% of N-STEMI patients develop cardiogenic shock1. Although it is recognised that an invasive approach is the recommended strategy for the majority of patients, achieving guideline-mandated timelines for angiography and revascularisation does not appear to be a strict goal in many units and understanding who needs earlier intervention even less considered. In this respect, and when contrasted to the unified approaches to STEMI (simple diagnosis to time-mandated intervention), N-STEMI remains very much the “Cinderella” of ACS.
It is true that randomised clinical trials have helped to define the appropriate range of pharmacological approaches and have outlined revascularisation strategies in N-STEMI, leading to improved patient outcomes2. Despite this, longer-term clinical event rates following N-STEMI remain unacceptably high, with registry data showing evident equivalence to STEMI (Figure 1), while other reports suggest even worse outcomes compared with STEMI3.
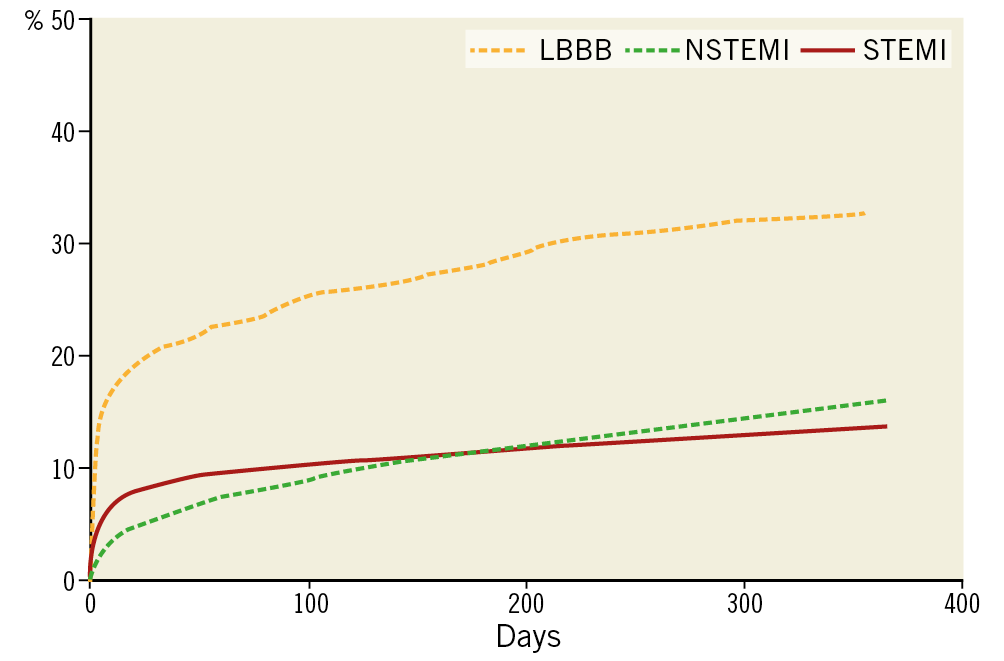
Figure 1. Mortality following ACS. Data extracted from the SWEDEHEART registry (courtesy of Professor Stefan James, with his permission).
In this study of “older” patients (median age 75 years) up to two years, the unadjusted cumulative incidence of all-cause mortality (16.0% versus 19.8%; p<0.001) and the composite outcome (21.9% versus 27.9%; p<0.001) was lower for STEMI patients. Even after adjustment there was little difference in hard endpoint outcomes.
Many interested in N-STEMI believe a number of key areas require addressing in order to improve clinical outcomes further. There have been opinions expressed that more urgent intervention could be the key. Providing a “STEMI-like approach” to all N-STEMI patients is clearly logistically and economically impossible for most countries, and also unnecessary since the prognostic natural history is variable. A better understanding of this natural history by ensuring optimal diagnostic certainty, using risk stratification and, where needed, undertaking randomised trials to test interventional strategies in the contemporary era would help to tailor management strategies.
In this opinion piece we discuss these aspects of N-STEMI, which we consider to be the “Cinderella” of the ACS spectrum – the term “Cinderella” is often used in English to describe something that is given less attention than it deserves. Dr Tom Kite, Research Fellow, highlights areas of existing clinical uncertainty around N-STEMI to obtain insights from two experienced clinician scientists, Professor Tony Gershlick and Professor Bernard Gersh.
1. DIAGNOSTIC DIFFICULTIES
Tom Kite: The recent publication of the fourth universal definition of myocardial infarction has placed emphasis on the separation of the entities myocardial infarction (MI) and myocardial injury, in part due to the widespread implementation of high-sensitivity troponin (hs-Tn) and its ability to detect more subtle injury to the myocardium4. The increase in sensitivity is good of course, as theoretically fewer cases will be missed. However, use and subsequent interpretation of hs-Tn, including application of the 99th centile, continues to perplex clinicians. Given the sometimes ambiguous clinical presentation of N-STEMI, with perhaps a lack of discriminating electrocardiographic (ECG) changes as compared to STEMI, hs-Tn appears to fail to provide the robust diagnostic accuracy that physicians require to confirm type 1 MI and exclude type 2 MI. Professor Gersh, how should we approach this issue?
Bernard Gersh: The fundamental concern is that many clinicians use hs-Tn as the central tenet for confirming (or “ruling in”) NSTEMI. Algorithmic risk modelling has been shown to be of great value and highly sensitive in the diagnosis of MI, as recently demonstrated by a risk-assessment tool integrating hs-Tn I or T concentration at presentation, and its dynamic change during serial sampling, to estimate the probability of MI and 30-day outcomes5. Yet, poor specificity associated with hs-Tn leads to poor predictive value. As such, hs-Tn has far greater value in “ruling out” myocardial injury (indeed a single undetectable troponin at >3 hours after onset of symptoms with a non-ischaemic electrocardiogram equates to a negative predictive value of >99.3%)6. However, this high diagnostic accuracy does not help to make a robust diagnosis of N-STEMI (“ruling in”). The High-STEACS trial confirmed that the introduction of high-sensitivity troponin, and use of the 99th centile as a diagnostic threshold, has resulted in the identification of greater numbers of patients with myocardial injury or infarction, but has failed to lead to a reduction in subsequent events7.
Tony Gershlick: Yes, I agree. Too often in clinical practice N-STEMI is diagnosed on a high Trop. In the UK, data from the National Institute for Cardiovascular Outcomes Research (NICOR) confirm that 85% of patients presenting with so-called “N-STEMI” undergo angiography8, whilst other data suggest that only 50% of those undergoing angiography proceed to intervention2. The implication is that many patients are being diagnosed as N-STEMI on the basis of the hs-Tn, but the diagnostic yield of angiography in terms of significant coronary stenoses is low. Questions have been raised regarding the use of the 99th centile alone as a threshold for diagnosis, not least since the manufacturer-provided reference ranges are derived from healthy individuals and will differ widely from the acutely unwell patient population presenting to hospital. A recently published study of 20,000 consecutive patients attending hospital found that the “true” 99th centile was more than six times the manufacturer-derived value, casting further doubt on the application of arbitrary cut-offs9.
Bernard, what about changing the definition of type 2 MI, somehow emphasising that troponin reflects myocardial injury rather than infarction? We all see patients with non-specific cardiovascular-like symptoms and raised troponin ending up in our cath labs. Certainly, the history and clinical context are critical. An elderly patient with a chest infection, atrial fibrillation, and funny chest pains should be considered carefully before going to the lab on the basis of their raised hs-Tn.
Bernard Gersh: Yes, we need to emphasise that the diagnosis of type 2 MI remains a clinical diagnosis based upon the history, ECG changes and a change in troponin levels compared to baseline. I do not think that the definition of type 2 MI needs to be changed, but what needs to be emphasised is that the definition refers to myocardial injury as opposed to infarction, and that there are multiple causes of myocardial injury. Persistently elevated troponin levels are not a false positive but should always prompt a search for other causes, not least when obstructive coronary disease is excluded, since they indicate a potentially adverse prognosis.
2. RISK STRATIFICATION
Tom Kite: A question for you both. We know that utilisation of risk scores to predict future clinical events is recommended in international guidelines, although there are few if any randomised trials that provide supporting data10,11. That said, one study has demonstrated that risk assessment based on clinical intuition is heterogeneous, and potentially associated with suboptimal provision of care as compared to objective assessment12. The Global Registry of Acute Coronary Events (GRACE) score has been used in >300 publications, is a well-validated risk stratification criterion, and has been shown to provide the most robust prediction of long-term outcomes despite its underuse in routine clinical practice13. So, should we risk stratify all N-STEMI? Just how valid or robust is risk stratification taking account of the fact that so few centres routinely perform this in everyday practice?
Tony Gershlick: Yes, it is a great question. It seems intuitive to see how “at risk” your patient is, but it is not common practice despite being given a high (IB) recommendation in the ESC Guidelines. The level of evidence B is explained by the lack of randomised trials. I guess the fact that risk stratification criteria are not systematically used in clinical practice may be due to clinicians’ perceptions that they are difficult to apply or that you need data at the time of admission that are not available – also wrong. Maybe clinicians just don’t think it is important enough to do, not worth spending time on, or are just not trained to do it as part of their initial assessment. The updated GRACE 2.0 or “mini-GRACE” score may help: it has superior discrimination, is easier to use than the original GRACE score, and is accessible on mobile platforms via an app, making it suitable as an easy way of determining the quantitated risk at the bedside using simple clinical parameters14. A GRACE 2.0 score of >118 suggests higher risk, with a six-month mortality of >6% – easy. Indeed, recent prospective observational data have suggested that optimal use of guideline-mandated treatment for N-STEMI is associated with greater survival gains, but higher-risk patients as defined by their GRACE score, surprisingly, receive optimal treatment less frequently15. However, when guideline-mandated treatment was appropriately given, there was a significant association with longer-term survival15. Given that the widespread use of invasive coronary strategies has provided the greatest effect on observed reduction in mortality in N-STEMI over recent years2, I think that risk stratification scores should be used routinely to assist in deciding who is at highest risk and guide the optimal therapy and even timing of catheter laboratory intervention. Bernard, any thoughts?
Bernard Gersh: Yes, I agree. I also think that using risk scores can be very helpful in trial design by enriching the patient population and ultimately keeping the sample size within reasonable limits. Furthermore, we should make it really easy for those working in acute admission units to access the calculators and complete risk scoring at point of entry into the system, and that use of such paradigms to determine risk should be considered part of the standard routine assessment of all ACS patients. The electronic medical record may be helpful in increasing the use of risk scores without being overly time-consuming.
3. OPTIMAL TIMING OF ANGIOGRAPHY
Tom Kite: So, another area of current contention and interest is centred on how we should act on the results of risk discrimination. Who should we take to the cath lab and when? Most know that current European and American guidelines advise an invasive strategy within 72 hours of admission for intermediate-risk N-STEMI, and within 24 hours of admission in high-risk cases (as defined by change in troponin, dynamic ECG changes, GRACE >140)10,11. However, these are proving difficult targets to achieve, as evidenced by recent UK data demonstrating that only 56% of patients underwent angiography within the recommended 72-hour time frame8. Maybe it is about focus and trying to ascertain whether we can identify intermediate- or higher-risk patients who may need more urgent invasive investigation with a view to intervention? Can you provide insights into the current evidence base that informs guideline recommendations of timing of angiography in N-STEMI?
Tony Gershlick: Data published to date have so far failed to demonstrate any difference in hard clinical endpoints between an earlier invasive strategy versus a deferred strategy in all-comer N-STEMI patients (although the definitions of “early” angiography, inclusion criteria, and endpoints in these historic randomised trials are highly variable). Importantly, this strategy was deemed safe with a lower risk of recurrent ischaemia16. However, in a 1,000-patient substudy of the TIMACS trial it was shown that in higher-risk patients (as defined by a GRACE score >140) an “early” invasive strategy (median = 14 hours) significantly reduced the risk of the composite endpoint of death, MI and stroke at six months by 35.0% (HR 0.65, 95% CI: 0.48-0.89, p=0.006)17. More recently, the VERDICT trialists analysed outcomes in a pre-specified subgroup with GRACE score >140, and again there appeared to be significant benefit with earlier intervention (median time of randomisation to angiography = 4.7 hours) – composite of death, MI, admission for heart failure, and admission for refractory ischaemia at 4.3-year follow-up for early intervention (HR 0.81, 95% CI: 0.67-1.01, p=0.023)18 (Figure 2).
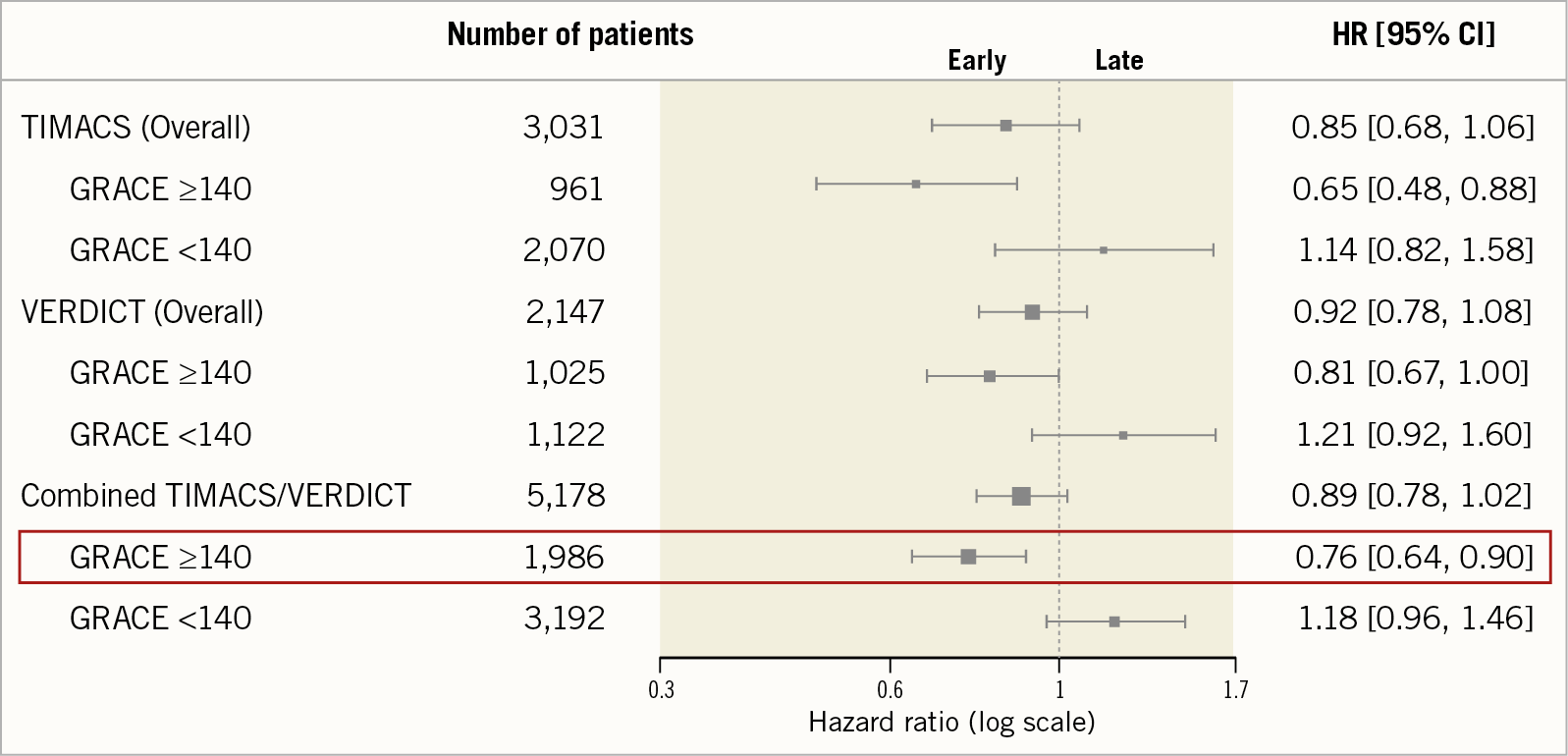
Figure 2. Subgroup analysis of high-risk patients (GRACE score >140) in TIMACS, VERDICT, and combined trial data for the respective primary outcomes.
Bernard Gersh: We need to state that both these a priori defined substudies, despite demonstrating benefit for their respective composite outcomes, were underpowered for individual hard clinical endpoints and, importantly, the primary outcome of both trials in the overall population was neutral. Pre-specified subgroup analyses are prone to confounding, thus confirmatory evidence in the form of a prospective, appropriately powered randomised trial in a higher-risk N-STEMI population is required. Some centres are developing acute N-STEMI pathways based on these subgroup data, but these could easily have the potential to impact adversely on established STEMI pathways, with nether sufficient scientific evidence of efficacy nor cost-effectiveness currently being available. Surely we should have more robust data before we rush perceived higher-risk N-STEMI patients to the cath lab in a STEMI-like manner, with all the potential impact this could have on staff working/burn-out, logistics, and service delivery?
4. NSTEMI PATHWAYS
Tom Kite: Following on from that point, what views do you have on designing N-STEMI pathways similar to the STEMI pathways that have proven so effective? Maybe we should have “ACS system” pathways? I am told by senior clinicians that previously patients with N-STEMI could wait days, perhaps even weeks, before receiving their diagnostic intervention. We recognise from UK audit data that inter-hospital transfer adds a full 24 hours to patients arriving in the cath lab19. Is this acceptable, pragmatic, or just how it is?
Bernard Gersh: Good practice I think involves accurate diagnosis, robust risk stratification, and appropriate timing of angiography. Therefore, these must be issues to consider in the contemporary management of N-STEMI. If this requires higher-risk patients being part of rapid investigation ACS pathways, then so be it. Such considerations provide further justification for a definitive randomised trial to identify accurately those specific patients who are most likely to benefit from expedited intervention.
Tom Kite: Yes, of course, but, taking account of the variable presentation of the syndrome, how do we best pull these things together to improve current N-STEMI care and patient pathways? At EuroPCR 2019, sessions discussing the implementation of “rapid” pathways for high-risk N-STEMI were held, with speakers promoting these as “evidence-based” to improve patient outcomes, despite the lack of a prospective randomised trial. Is this right?
Tony Gershlick: I think that I would mention again that troponin-determined N-STEMI is part of a risk stratification schema, but not the entire picture. Again, risk scores may help. Moreover, as I stated earlier, while subgroup analyses as per TIMACS and VERDICT are better than no data, they do have their weaknesses. Whether they are sufficient to base strategic pathway development on is a moot point, albeit, in my view, it is the right direction of travel. Four considerations must be taken into account with regard to N-STEMI care: ease of robust diagnosis, risk-scored case selection, adherence to clinical guidelines, and the need for revised and improved networks to meet increasing demand. We also need to understand that clinical guidelines reflect a consensus and understand the limitations of the available evidence and lack of a randomised trial in the current era.
The poor discriminatory value of hs-Tn for identifying N-STEMI, and issues with “troponitis” must be addressed, with continued emphasis on identification of novel biomarkers (perhaps as part of a biomarker panel in addition to hs-Tn), to increase specificity and predict better those patients who require PCI. Once we have selected the right patients then it must be proven that acting on them more expeditiously is critical. Bernard and I agree that such developments would facilitate compliance with guideline-mandated timing and help to ensure that the correct patients receive the appropriate treatment, and at the optimal time. However, diagnosis issues aside, “rapid” pathways for so-called higher-risk patients do require serious consideration, given the suggestion of benefit described in the evidence, albeit as you say in substudy data. I do think that without more robust data there lie significant challenges with managing resources, flexible working patterns, and cost efficacy – all require careful attention. A restructure of pathways similar to the STEMI revolution of 20 years ago is a worthy aspiration of course but only for those who can robustly be shown to benefit.
Tom Kite: So, what can we conclude?
All: If we are to draw any conclusions from this discussion, it would be our view that the undoubted success of STEMI pathways and networks has not yet been translated into the N-STEMI arena, and maybe we should focus our efforts on better management of this “Cinderella” of ACS. Given that N-STEMI comprises the majority of patients with ACS, and may carry an adverse prognosis in some, then establishment of contemporary N-STEMI pathways is likely to be of value. However, while decisions on diagnosis and early invasive management can now be made much earlier with modern biomarker detection, we must ensure that appropriate patients are selected for any strategy that involves early STEMI-like transfer and timely intervention. Further randomised trials may help to support the value of early risk stratification and confirm the perceived benefit of very early intervention in higher-risk patients. Robust cost-efficacy analyses and careful consideration must be given before we can support the current trend of revising and restructuring ACS pathways.
Conflict of interest statement
The authors have no conflicts of interest to declare.

