Abstract
Aims: An intracoronary electrocardiogram (IC-ECG) is a sensitive method to detect local myocardial ischaemia. We investigated the prevalence of IC-ECG ST-segment elevation (STE) with respect to culprit lesion location in patients with non-ST-segment elevation myocardial infarction (NSTEMI) and its relationship with elevated levels of cardiac biomarkers.
Methods and results: We examined 87 NSTEMI patients who underwent IC-ECG recording by locating the insulated polymer-coated guidewire distal to the culprit lesion before percutaneous coronary intervention (PCI). Cardiac biomarkers were serially examined. IC-ECG STE was observed in 24 patients (27.6%) before PCI, and was significantly more frequent in patients with LCx culprit lesions (LAD vs. LCx vs. RCA, 12.1% vs. 53.3% vs. 16.7%; p<0.001). Peak cardiac troponin I (cTnI) values were associated with IC-ECG STE, ejection fraction (EF), cTnI values on admission, and type B2/C lesions. In multivariate analysis, IC-ECG STE (odds ratio [OR], 5.04; 95% confidence intervals [CI]: 1.51-16.85; p=0.009), and EF (OR, 0.95; 95% CI: 0.90-1.00; p=0.043) were predictors of greater peak cTnI values.
Conclusions: IC-ECG STE was not uncommon in NSTEMI patients, particularly those with LCx culprit lesions. IC-ECG monitoring before PCI may help identify NSTEMI patients with high risk of greater myocardial injury.
Introduction
Studies investigating ST-segment elevation myocardial infarction (STEMI) have described an uneven distribution of culprit lesion locations, with the majority occurring in the left anterior descending artery (LAD) and the right coronary artery (RCA)1,2. Recent randomised clinical trials of STEMI have also shown that the left circumflex coronary artery (LCx) is the least frequent location of culprit lesions in enrolled patients3,4. However, there appears to be a more uniform distribution among patients with non-ST-segment elevation myocardial infarction (NSTEMI)5,6. One possible explanation is that left circumflex arterial occlusion is less likely to be diagnosed as STEMI on a standard 12-lead electrocardiogram (ECG), compared with LAD or RCA occlusion. Although the ECG diagnostic criteria for STEMI are well established7, the sensitivities of these criteria may be suboptimal8,9. In a recent study using contrast-enhanced magnetic resonance imaging as the criterion standard, the European Society of Cardiology/American College of Cardiology/American Heart Association consensus ST-segment elevation criteria had only 50% sensitivity for acute myocardial infarction (MI)9. NSTEMI and STEMI have traditionally been considered two different entities; however, both exhibit similar outcomes with common pathophysiology, and similar risk factors, pathogenesis, and prognosis10,11. Thus, it may be that differences in the distribution of infarct-related arteries identified in STEMI trials occur because LCx occlusion is often missed by routine 12-lead ECG. Thus, patients with LCx-culprit MI manifested as NSTEMI may often fail to meet the inclusion criteria for STEMI. In other words, insufficient sensitivity of standard surface 12-lead ECG, particularly for LCx-culprit lesion NSTEMI, may result in suboptimal detection of STEMI-equivalent patients who require emergent revascularisation therapy.
Unipolar intracoronary ECG (IC-ECG) recording from a guidewire electrode has been shown to be more sensitive and reliable in detecting regional myocardial ischaemia during coronary intervention than standard surface ECG12-16. We assessed the hypothesis that IC-ECG ST-segment elevation in the area at risk in patients with NSTEMI would be detected in a significant proportion of patients with LCx culprit lesions, compared with other coronary artery lesions.
Furthermore, we hypothesised that IC-ECG ST-segment status before percutaneous coronary intervention (PCI) could provide important information to assess myocardial injury in patients with NSTEMI.
Methods
STUDY SAMPLE
We prospectively studied 110 patients with NSTEMI admitted to Tsuchiura Kyodo Hospital from November 2010 to October 2011. This hospital is the primary medical centre for a population of 150,000 and is located in the suburban region north of Tokyo. Patients were eligible if they had ischaemia symptoms that were increasing or occurring at rest for more than 10 minutes within the past 12 hours; unequivocal changes on an admission ECG (transient or persistent pathological ST-segment depression >0.1 mV, or T-wave inversion in at least two adjacent leads without pathological Q-waves); elevated cardiac biomarkers; and no contraindication for PCI. Clinical exclusion criteria were age <21 years; chest pain suspected not to be caused by coronary artery disease (CAD); STEMI; history of MI; recent PCI; renal insufficiency with a baseline serum creatinine level >1.8 mg/dL (133 μmol/L); inability to provide informed consent; concomitant non-cardiac life-threatening disease; severe haemodynamic impairment or cardiogenic shock at admission; and other significant cardiac disease. No upper age limit was set. All patients were required to undergo coronary angiography within 48 hours of admission, as an early routine invasive strategy, with revascularisation by stenting as appropriate. Of 110 patients initially selected for the study, eight patients with multivessel disease were excluded because culprit lesions could not be identified; six were excluded because of multivessel CAD or left main CAD requiring coronary artery bypass grafting; five because of absence of significant CAD according to the angiogram; three for major (>1.5 mm) side branch occlusion after PCI; and one for coronary anatomy unsuitable for stent implantation. NSTEMI was diagnosed on the basis of the cardiac troponin I (cTnI) value at admission17. Thus, 87 patients (66 men, 21 women) were included in the final study group. Patients received medical treatment according to the guidelines18. Platelet glycoprotein IIb/IIIa receptor inhibitors were not used because they are not available in Japan. This study complied with the principles set out in the Declaration of Helsinki. All patients gave written informed consent before catheterisation, and the study protocol was approved by the institutional ethics committee.
BIOCHEMICAL ANALYSIS
The Abbott Architect Troponin I assay (Abbott Diagnostics, Abbott Park, IL, USA) was performed with a detection limit of 0.01 ng/mL, and a coefficient of variation less than 10% at 0.032 ng/mL, as specified by the manufacturer. The upper reference limit (URL) was 0.20 ng/mL at our institution: this represented the 99th percentile of the distribution of a reference control group. Biochemical measurements were performed at admission, prior to PCI, within one hour of PCI completion, 6, 12, 18, and 24 hours after PCI, and at six-hour intervals thereafter if the values were still increasing. NSTEMI was diagnosed using a cut-off value of cTnI >0.20 ng/mL at admission sampling. CK-MB (creatine kinase MB) activity was determined using an immunoinhibition assay and confirmed by mass assay technique. The maximum value (peak cardiac biomarker level) was considered to represent the extent of myocardial damage.
ANGIOGRAPHIC ANALYSIS
Quantitative coronary angiography (QCA) analysis was performed using a CMS-MEDIS system (Medis Medical Imaging Systems, Leiden, The Netherlands). The minimum lumen diameter, reference diameter, and lesion length were measured in diastolic frames from orthogonal projections. Coronary flow was assessed according to the Thrombolysis In Myocardial Infarction (TIMI) flow grade19. The culprit lesion was determined using coronary angiograms and corroborated with information from the patient’s electrocardiogram and echocardiogram. Procedural success was defined as the angiographic criterion of <20% residual stenosis with TIMI 3 flow.
PERCUTANEOUS CORONARY INTERVENTION PROCEDURE
All patients received aspirin (200 mg) before and after PCI. Clopidogrel (loading dose 300 mg, followed by 75 mg/day) was administered before PCI and daily thereafter. All patients received an intravenous bolus injection of 10,000 IU of heparin and intracoronary isosorbide dinitrate (2 mg) before angiography, and an additional bolus of 2,000 IU heparin was given every hour if the procedure lasted for more than one hour. All PCI procedures were performed using a 6 Fr guiding catheter via the radial approach. The type of stent was selected at the operator’s discretion. All patients underwent coronary stent implantation with predilatation.
STANDARD AND IC-ECG RECORDING
Standard 12-lead ECG was recorded at admission, before PCI, at the end of PCI, and one hour and three hours later with a paper speed of 25 mm/s and a signal amplitude of 10 mm/mV. The position of the electrodes on the chest was marked to allow reproducible recordings. Standard 12-lead ECG was initially analysed by two expert cardiologists who determined patient diagnosis. IC-ECG was recorded by connecting the proximal tip of the 0.014 inch diameter guidewire (Hi-Torque Balance Middle Weight Universal; Abbott Vascular, Santa Clara, CA, USA) to a multichannel ECG recorder (RMC-4000 Cardio Master with EP amplifier system [JB400G]; Nihon Koden, Tokyo, Japan). The proximal shaft of this guidewire was coated with an insulated polymer cover, and a distal uninsulated component was used as a unipolar electrode. This system enables simultaneous recordings of surface ECG limb leads and chest leads (V1 to V6) during multichannel IC-ECG recordings. The data were stored digitally for offline analysis. IC-ECG and surface ECG were calibrated for a signal amplitude of 10 mm/mV, as previously described15,16. After the guidewire passed the stenosis and was positioned distally in the culprit vessel, and before IVUS examination, an intracoronary injection of 200 µg nitroglycerine was administered and IC-ECG was recorded (baseline IC-ECG recording). If the target lesion was located before a major vessel bifurcation, the guidewire tip was positioned in the major distal vessel. Intracoronary ST-segment changes were measured 60 milliseconds after the end of the QRS or QS complexes by digital magnification, approximated to the nearest 0.5 mm. The isoelectric line was considered the T-P segment preceding the QRS (or QS) complex. Three consecutive QRS complexes were analysed, and mean ST-shift values were calculated. ST-segment elevation of IC-ECG (IC-ECG STE) was defined as >0.2 mV ST-segment elevation compared with the corresponding isoelectric line. IC-ECG was analysed by two independent investigators who were blinded to the clinical presentations. When there was discord between the observers, a consensus reading was obtained.
STATISTICAL ANALYSIS
SPSS 14.0 (SPSS Inc., Chicago, IL, USA) was used for baseline descriptive analyses. Normality of the data was verified by the Kolmogorov-Smirnov test. Categorical data were expressed as absolute frequencies and percentages and were compared using the χ2 or Fisher’s exact test, as appropriate. Continuous variables were expressed as mean±standard deviation for normally distributed variables and as median with interquartile range (IQR) for non-normally distributed variables, and they were compared using the Student’s t-test and Mann Whitney U test, respectively. Differences among the groups were analysed by the Kruskal-Wallis test. Post hoc paired comparisons were performed after the Kruskal-Wallis test using pairwise comparison of subgroups according to Conover20, with MedCalc (MedCalc Software, Mariakerke, Belgium). The relationships among peak cTnI values (dependent variable), clinical and angiographic characteristics, baseline IC-ECG STE, and other potential confounders were assessed using multivariate logistic regression analysis (stepwise forward method) to determine whether baseline IC-ECG STE was still associated with peak cTnI values. Associated variables on univariate analysis (p<0.1) were included in the model. We considered p<0.05 to indicate statistical significance.
Results
IC-ECG recording was successful and interpretable in all patients, and no serious complications were observed during IC-ECG recordings. Of 87 patients who underwent pre-procedural IC-ECG recordings, no-reflow phenomenon (TIMI 0-2 flow) occurred in seven after PCI. Three of them presented pre-procedural IC-ECG STE. The remaining 80 patients were successfully treated with stenting.
PREVALENCE OF BASELINE IC-ECG STE ACCORDING TO CULPRIT LESION
Figure 1 shows the prevalence of baseline IC-ECG STE according to culprit lesion. Lesion locations were as follows: 33 in the LAD, 30 in the LCx, and 24 in the RCA. IC-ECG STE was observed in 24 patients (27.6%). Baseline IC-ECG STE was significantly more frequent in patients with LCx culprit lesions (LAD vs. LCX vs. RCA, 4/33 [12.1%] vs. 16/30 [53.3%] vs. 4/24 [16.7%], respectively; p<0.001).
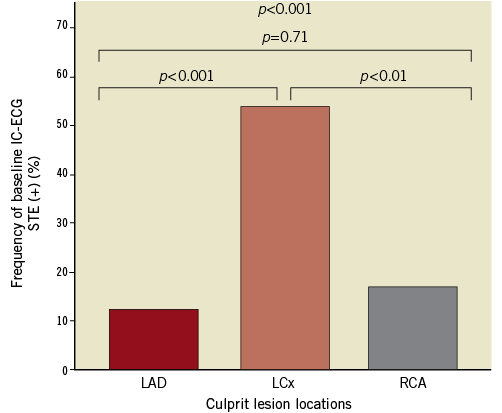
Figure 1. The frequency of baseline IC-ECG STE and culprit lesion locations. The frequency of baseline intracoronary electrocardiogram (IC-ECG) ST-segment elevation (STE) was significantly greater in patients with lesions in the left circumflex coronary artery.
PATIENT CHARACTERISTICS, PRESENTING FEATURES, AND ANGIOGRAPHIC FINDINGS STRATIFIED BY IC-ECG STE BEFORE PCI
The baseline characteristics, presenting features, and angiographic findings for patients with and without IC-ECG STE before PCI are summarised in Table 1 and Table 2. Representative cases with IC-ECG recordings before PCI are shown in Figure 2. There were no significant differences in baseline clinical characteristics among patients with IC-ECG STE and those without IC-ECG STE. Peak cardiac biomarker levels were significantly higher in patients with IC-ECG STE than in those without (CK-MB, 91 [IQR 44-266] vs. 36 [IQR 19-61], p<0.01; cTnI, 32.5 [IQR 18.1-107.8] vs. 9.1 [IQR 2.1-23.5], p<0.01, respectively) (Figure 3). There was no significant difference in the time interval from the symptom onset to the blood sampling between patients with IC-ECG STE and those without (p=0.34). Also, no difference in the time interval from the onset to peak biomarker values was noted between patients with IC-ECG STE and those without. When seven patients with no-reflow after PCI were excluded from the analysis, the elevated biomarker levels were similarly higher in the baseline IC-ECG STE group (CK-MB, 104 [IQR 45.5-270] vs. 36 [IQR 18-62], p<0.01; cTnI, 36 [IQR 18.8-113.5] vs. 9.8 [IQR 2.1-24.2], p<0.01, respectively). Twenty-nine lesions (33.3%) exhibited an occluded (TIMI 0-1) culprit vessel at the time of angiography. Pre-PCI IC-ECG STE showed a non-statistically significant trend in association with worse coronary flow at baseline. There was no significant difference between the two groups in the level of collateral coronary flow at baseline coronary angiogram. When patients were grouped into quartiles based on peak cTnI values, the frequency of baseline IC-ECG STE significantly increased according to peak cTnI value (Figure 4).
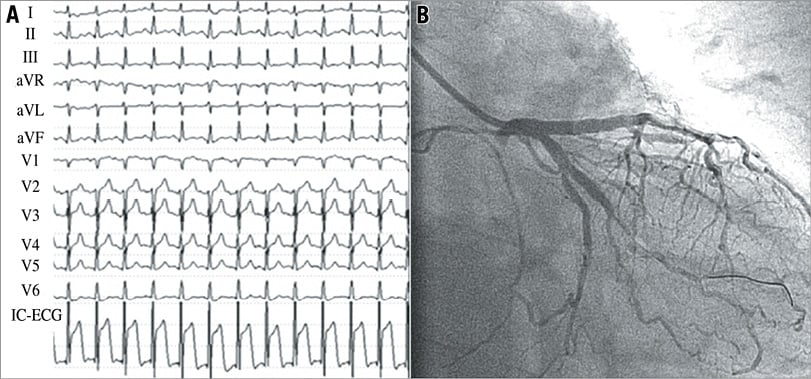
Figure 2. Representative surface ECG and IC-ECG recordings in a patient with a left circumflex coronary artery culprit lesion. Surface electrocardiogram (ECG) shows no ST-segment elevation, and intracoronary electrocardiogram (IC-ECG) shows significant elevation of the ST-segment (A). The coronary flow grade was Thrombolysis In Myocardial Infarction (TIMI) 2 at baseline (B).
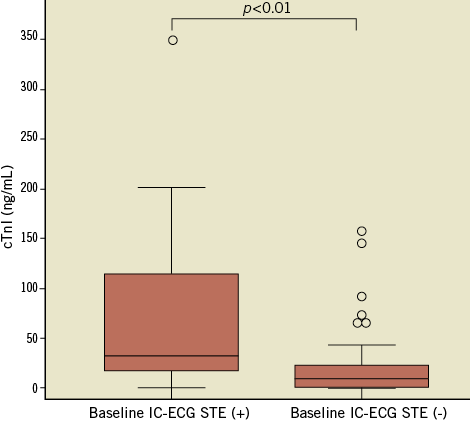
Figure 3. Peak cTnI values and baseline IC-ECG STE. The peak cardiac troponin I (cTnI) values were significantly greater in the patients with baseline intracoronary electrocardiogram (IC-ECG) ST-segment elevation (STE).
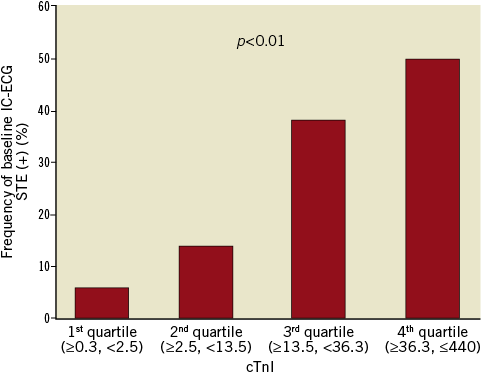
Figure 4. The frequency of baseline IC-ECG STE and peak cTnI values. Patients were grouped into quartiles based on peak cardiac troponin I (cTnI) values. The frequency of baseline intracoronary electrocardiogram (IC-ECG) ST-segment elevation (STE) increased significantly according to peak cTnI values.
PREDICTORS OF GREATER PEAK cTnI VALUES
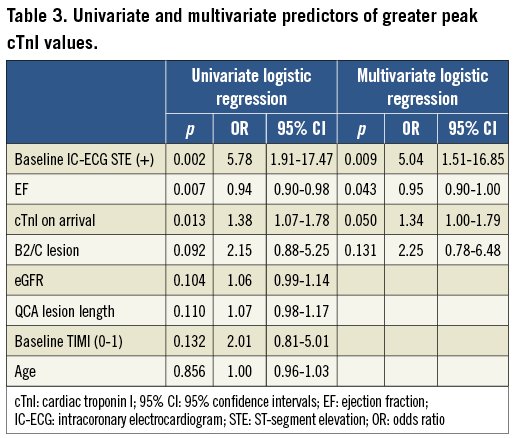
The results of univariate and multivariate logistic regression analyses for greater peak cTnI values (more than the median value) are given in Table 3. In multivariate analysis, baseline IC-ECG STE (OR, 5.04; 95% CI: 1.51-16.85; p=0.009) and ejection fraction (EF) at presentation (OR, 0.95; 95% CI: 0.90-1.00; p=0.043) remained independent predictors of greater peak cTnI values.
Discussion
Our major findings are as follows: (1) baseline IC-ECG STE was observed in 27.6% of patients with NSTEMI on surface ECG; (2) baseline IC-ECG STE was significantly more frequent in patients with LCx culprit lesions (53.3%) than in those with LAD or RCA culprit lesions (12.1% and 16.7%, respectively); (3) cardiac biomarker levels were significantly more elevated in patients with IC-ECG STE than in those without IC-ECG STE.
Acute coronary occlusion is associated with a high risk of adverse outcomes among patients with myocardial infarction21,22. The presence of ST-segment elevation on standard surface ECG is the most common criterion used in clinical practice to identify patients with acutely occluded coronary arteries, and these patients should be treated by emergent reperfusion therapy. However, it is not uncommon to encounter a patient who, based on history and laboratory findings, is a candidate for primary PCI, but has an ECG that does not provide an evidence-guided pathway to emergent angiography and revascularisation. The sensitivity of 12-lead surface ECG for ST-segment elevation is low, particularly with LCx occlusion2,9,23,24. Pande et al and Berry et al performed surface and intracoronary electrocardiography during PCI25,26. Of 19 patients who had intracoronary lead ST-segment elevation with balloon inflation of LCx, only 32% manifested ST-segment elevation on the surface ECG, and 47% had no ST-segment deviation. This was in contrast to the almost 90% of patients with LAD or RCA balloon inflation who had shown ST-segment elevation on surface ECG. Our results showed that, in patients with NSTEMI, IC-ECG STE was observed in 53.3% who had LCx culprit lesions, in contrast to 12.1% and 16.7% of those with LAD and RCA culprit lesions. To our knowledge, this is the first study to show that IC-ECG STE prevalence differs according to lesion location and is detected more frequently in NSTEMI patients with LCx culprit lesions. Intracoronary unipolar ECG reflects local electrical events of the myocardium near the tip of the guidewire. The magnitude of the current in the injured region during acute myocardial ischaemia is greatly influenced by its distance from the recording electrode. Thus, an electrode placed on the surface of the heart is more sensitive and accurate than standard ECG leads in detecting the ischaemic ST-segment changes13,27. The present results suggest that myocardial ischaemia that causes ST-segment elevation measured by an electrode placed directly on the ischaemic myocardium is not uncommon in NSTEMI patients, particularly when culprit lesions are located in the LCx. Consequently, it is reasonable to conclude that the underrepresentation of circumflex vessels in STEMI clinical trials may be due to our reliance on standard surface ECG25,26. Further research is needed to ensure that STEMI-equivalent NSTEMI patients who are not identified by standard risk stratification approaches (based on 12-lead ECG) benefit from proper angiography and reperfusion. The multichannel ECG recorder system (72 channels including standard 12-lead recording) used in the present study can monitor and digitally store the simultaneous recordings of 12-lead ECG and multiple intracoronary ECGs, as well as extended lateral chest leads. This system may help identify candidates for emergent revascularisation in future NSTEMI studies by determining the relationship between IC-ECG, extended chest leads such as V7-V9, and standard 12-lead ECG.
In our study, ST-segment status on intracoronary ECG was associated with myocardial injury. Peak cardiac biomarker levels (CK-MB and cTnI) were significantly higher in patients with IC-ECG STE than in those without IC-ECG STE. On logistic multivariate analysis, baseline IC-ECG STE and EF at presentation were predictors of greater peak cTnI values. Monitoring the IC-ECG ST-segment in NSTEMI patients using a guidewire before PCI is a fast, simple, safe and inexpensive diagnostic method for assessing myocardial injury, and it may predict adverse outcomes28. There is convincing evidence that IC-ECG is more sensitive than surface ECG in detecting ischaemia, and it represents local epicardial electrical activity well, particularly in the LCx. However, IC-ECG may miss ischaemic changes because of its small interrogation area. At this time, there is no standardised method for recording IC-ECGs. Given its feasibility and lack of additional cost in PCI settings, further studies should be conducted to determine whether a strategy employing IC-ECG can benefit NSTEMI patients. The present study suggests that late diagnosis and treatment of NSTEMI with LCx culprit lesions probably results in greater myocardial injury. Efforts to detect acute coronary events must be intensified, particularly with regard to the LCx, which may benefit from early revascularisation.
Limitations
This study had a number of limitations. First, although the data were collected prospectively, this was a retrospective, single-centre analysis based on a relatively small number of patients and was subject to the limitations of such analyses. Core laboratory analysis of the angiograms and ECGs was not performed. Second, the definition of ST-segment elevation was arbitrarily determined at our institution, because there is no established definition of the ST-segment elevation on IC-ECG that indicates local myocardial ischaemia. This should be addressed in future studies.
Conclusions
IC-ECG STE was not uncommon in NSTEMI, particularly when culprit lesions were located in the LCx. IC-ECG monitoring prior to PCI may help identify NSTEMI patients with high risk of greater myocardial injury.
Conflict of interest statement
The authors have no conflicts of interest to declare.

