Abstract
Aims: Evidence regarding the optimal treatment of non-culprit lesions detected during primary PCI is lacking. Our aim was to investigate whether early invasive treatment improves left ventricular ejection fraction (EF) and prevents major adverse cardiac events (MACE).
Methods and results: Of 121 patients with at least one non-culprit lesion, 80 were randomised to early FFR-guided PCI (invasive group), and 41 to medical treatment (conservative group). Primary endpoint was EF at six months, secondary endpoints included MACE. In the invasive group, early angiography was performed 7.5 days (5-20) after primary PCI. Forty percent of the non-culprit lesions did not show haemodynamic significance (FFR > 0.75). Subsequent PCI of at least one non-culprit lesion was performed in 52%, PCI without preceding FFR was performed in 8% and elective CABG was done in 4%. No in-hospital events occurred in the conservative group. After six months, EF was comparable (59±9% vs. 57±9%, p=0.362), and there was no difference in MACE between invasively and conservatively treated patients (21 vs. 22%, p=0.929).
Conclusions: An invasive strategy towards non-culprit lesions does not lead to an increase in EF or a reduction in MACE. The functional stenosis severity of non-culprit lesions is frequently overestimated.
Introduction
Patients with multivessel disease form a subgroup at high risk for major adverse cardiac events (MACE) in the first year after primary percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI), with a reported incidence of 14.5% of MACE in patients with single vessel disease, compared to
19.5% and 23.6% in those with two- and three-vessel disease, respectively1.
Part of this risk may be attributed to the unfavourable risk profile of these patients, including higher age and the presence of diabetes. However, even when outcome is corrected for these associated risk factors, mortality in patients with multivessel disease is still increased almost two-fold after five years2.
This increase in risk that can not be explained by comorbidity may be related to the fact that multivessel disease inhibits recovery of left ventricular function3 and even promotes left ventricular dilatation after myocardial infarction4. Furthermore, it has been shown that the presence of multiple complex plaques is related to more adverse cardiac events during follow-up5.
Based on these data, early additional revascularisation after primary PCI may have a favourable effect on patient outcome. This potential benefit must be offset against the risk of an additional invasive procedure early after STEMI.
Currently, it is recommended by the guidelines to defer treatment of non-culprit lesions until about six weeks after hospital discharge if ischaemia is documented6,7. This recommendation is based on a non-randomised study that demonstrated a worse outcome in patients that underwent multiple procedures early after STEMI8. More recent reports have suggested that early additional revascularisation may be safe and beneficial9-11. However, additional interventions were not ischaemia-guided in these observational studies, and selection bias may have influenced their results.
In this randomised trial, we hypothesised that early ischaemia-guided revascularisation after primary PCI would result in an improvement of global left ventricular function and less cardiac events during follow-up when compared to a more conservative treatment strategy.
Methods
Patients
Patients were recruited in a single tertiary referral centre in The Netherlands. The study was approved by the Medical Ethics Committee of the hospital. Written informed consent was obtained in all patients.
Patients with multivessel disease who underwent successful primary angioplasty for STEMI were candidates for the study. Successful PCI was defined as a residual diameter stenosis of < 50% and TIMI 3 flow. Multivessel disease was defined as one or more significant stenoses in at least two major epicardial coronary arteries, or the combination of a side branch and a main epicardial vessel provided that they supplied different territories12. A significant stenosis was defined as a diameter stenosis of at least 50% in luminal diameter (in at least one view, on visual interpretation or preferably by QCA). The reference diameter adjacent to the lesion to be treated had to be at least 2.5 mm. Patients were excluded from the study if they had an urgent indication for additional revascularisation, were > 80 years old, had a chronic occlusion of one of the non-infarct related arteries, prior CABG, left main stenosis of 50 % or more, restenotic lesions in non-infarcted arteries, chronic atrial fibrillation, limited life-expectancy, or other factors that made complete follow-up unlikely.
The indication for an additional revascularisation procedure outside the protocol was determined by an expert panel of interventional cardiologists and thoracic surgeons (at least one of each discipline).
Patients fulfilling both in- and exclusion criteria were randomised to one of the two following strategies (Table 1).
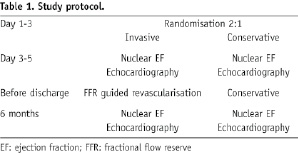
INVASIVE TREATMENT
In this treatment group, ischaemia-guided additional revascularisation was performed during the in-hospital phase after primary PCI or in an out-patient setting but no later than three weeks after STEMI. After repeat coronary angiography, fractional flow reserve (FFR) was measured in the vessels with a significant stenosis and also in the infarct-related artery (IRA) if restenosis was present. If the FFR was compatible with ischaemia (FFR <0.75), PCI of the stenosis was performed. Otherwise, the vessel was left untreated. In severe lesions (>90% stenosis), PCI was performed without preceding FFR measurement. Implantation of a stent and the type of stent (bare metal or drug-eluting) was left to the discretion of the operator.
CONSERVATIVE TREATMENT
In this group, further treatment after primary PCI was left to the treating physician. Aggressive revascularisation without symptoms was discouraged in this group. If symptoms did occur, a strategy of ischaemia-guided additional revascularisation was followed. Exercise testing, dobutamine stress echocardiography or myocardial scintigraphy were considered acceptable means to demonstrate ischaemia.
Randomisation was performed by means of a computer program. Patients were allocated to an invasive or conservative treatment in a 2:1 ratio respectively (see also power calculation).
In both groups, left ventricular function was evaluated before discharge (and before any additional interventions) using radionuclide ventriculography and echocardiography. These studies were repeated at six months.
Major adverse cardiac events (MACE) were scored in all patients and included death, nonfatal reinfarction and additional unplanned revascularisation procedures. Non-fatal reinfarction was defined as new Q-waves on the ECG or a new CK and CK-MB rise above the upper limit of normal. This included periprocedural infarctions in the invasive treatment arm.
Endpoints
Primary endpoint was ejection fraction assessed by radionuclide ventriculography at six months.
Secondary endpoints were:
– Change in ejection fraction (baseline to six months);
– Wall motion score, left ventricular end-systolic and end-diastolic volume and ejection fraction at six months as assessed by echocardiography;
– Major adverse cardiac events (MACE).
Endpoints were analysed as intention-to-treat, and also per protocol in patients who actually underwent conservative treatment or repeat coronary angiography and FFR measurements, respectively. Echocardiographic and radionuclide data were blinded to treatment allocation.
Studies and procedures
FFR MEASUREMENT
FFR measurements were used to determine the indication for an additional PCI. During coronary angiography, a 0.014 inch pressure monitoring wire (RADI Pressure™ wire, RADI Medical Systems AB, Uppsala, Sweden) was set to zero, calibrated, advanced through a guiding catheter, introduced in the coronary artery and positioned distal to the stenosis under investigation. Adenosine (140 µg/kg/min) was infused continuously through a 5 Fr sheath in the femoral vein to obtain maximal coronary blood flow, corresponding to minimal distal coronary pressure. After steady-state hyperaemia was achieved, FFR was calculated as the ratio of the mean distal intracoronary pressure measured by the pressure wire, and the mean arterial pressure measured through the coronary guiding catheter.
NUCLEAR EJECTION FRACTION
Radionuclide ventriculography was performed after randomisation but before any additional intervention and repeated after six months in all patients. Measurements were performed by the multiple-gated equilibrium method after in vivo labelling of red blood cells with 99mTc pertechnetate (800 MBq). A gamma camera (General Electric, Milwaukee, WI, USA) was used. The global ejection fraction (EF) was calculated using the General Electric eNTEGRA® software.
ECHOCARDIOGRAPHIC MEASUREMENTS
Echocardiograms were made after randomisation but before any additional intervention and repeated after six months of follow-up. Left ventricular end-systolic and end-diastolic volume were assessed using the modified biplane Simpsons’ rule and the ejection fraction was calculated from these values. Also, the echocardiographic wall motion score was assessed according to the American Society of Echocardiography13. Echocardiographic images were stored digitally on optical disk and on a separate videotape for each individual patient.
Medication
All patients were treated with aspirin, beta-blockade and an ACE-inhibitor unless contraindicated. Patients who received a stent were treated with clopidogrel 75 mg once daily for at least 30 days after a loading dose of 300 mg. Use of a glycoprotein IIb/IIIa inhibitor during or after angioplasty was left at the discretion of the operator.
Statistics and presentation of the results
Students’ t-test was used to assess statistically significant differences between continuous variables, Chi-square was used for differences between proportions. A two-sided p-value of < 0.05 was considered statistically significant.
POWER CALCULATION
A difference of 5% in ejection fraction was considered clinically relevant. With a standard deviation of 12%, a power of 80% and alpha of 0.05, 92 patients in both groups were needed to detect a significant difference at the p < 0.05 level. A 10% drop-out rate was taken into account. In addition, it was estimated that only about 50% of the lesions considered significant during primary angioplasty in fact would produce ischaemia during FFR measurement.14,15 Therefore, patients were randomised in a 2:1 ratio (invasive: conservative treatment). The aim was to include a total of 300 patients in the study.
Exclusion log
Patients with multivessel disease were randomised between day one and three after the primary angioplasty procedure. Patients with multivessel disease who were not randomised were entered in an exclusion log stating the reason for exclusion.
Results
Between June 2004 and February 2007, 952 patients with multivessel disease and STEMI treated with primary PCI were considered for entry in the study (Table 2).

A considerable number of patients could not be included because of participation in other primary PCI studies (199 patients) or logistical problems (159 patients, mostly early transfers to referring hospitals).
After application of in- and exclusion criteria,121 patients could be included and randomised in this period (average inclusion rate: 3.8 patients/months). To achieve a total inclusion of 300 patients, it was anticipated that the inclusion period had to be extended with another 47 months, up to a total of over 6.5 years. Because of the changes in clinical practice in this period (increase in the use of DES and IIb/IIIa inhibitors; changes in clopidogrel regimen, introduction of new drugs) this would result in an inhomogeneous study population, and therefore it was decided to stop the study prematurely in March 2007.
Baseline characteristics of the included patients (Table 3) were fairly well balanced between the two treatment groups, although hypertension and hypercholesterolaemia were more frequent in patients who were treated conservatively.
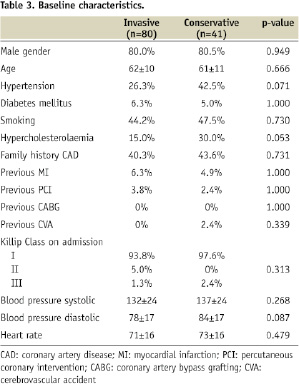
The prevalence of diabetes, heart failure and previous cardiac events was low in both groups. Most patients had two-vessel disease, and the RCA was the infarct-related artery in the majority of cases (Table 4).
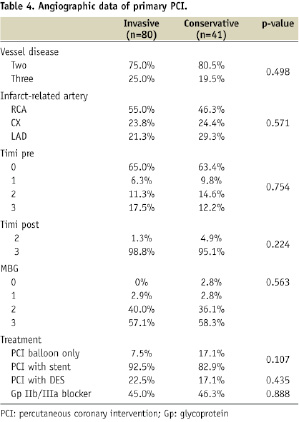
The number of non-culprit lesions per patient was comparable in both groups (1.3±0.5 vs. 1.2±0.4, p=NS). QCA was performed retrospectively in 103 non-culprit lesions (67%) and showed that the stenosis severity was slightly higher in the conservative group (60±8% vs. 52±12%, p=0.001).
Almost all patients had TIMI 3 flow after the procedure, and myocardial blush grade was 2 or 3 in over 90%. About 90% of the patients received a stent, of which 20% was drug-eluting. Almost half of the study population was treated with a glycoprotein IIb/IIIa inhibitor.
In the invasive group, repeat coronary angiography was performed in 76/80 patients (95%) at a median of 7.5 days (interquartile range 5 - 20) after primary PCI (Figure 1).
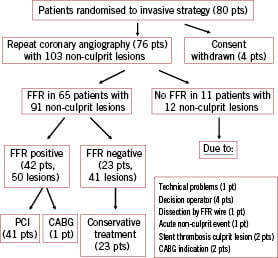
Figure 1. Repeat coronary angiography and FFR measurements.
These 76 patients had 103 non-culprit lesions. FFR was performed in 65/80 (81%) of the invasive patients who had 91/103 (88%) non-culprit lesions. FFR was negative in 41/103 lesions (40%) and positive in 50/103 lesions (49%); in the remainder of the lesions FFR was not performed (11%). Patients with negative FFR measurements only were treated conservatively (23/80, 29%). All but one of the patients with at least one positive FFR measurement (42/80, 53%) underwent subsequent PCI of at least one non-culprit lesion; one patient was treated by elective CABG.
Of the total invasive group, 42 patients had at least one PCI procedure of a non-culprit lesion after FFR, another six patients had PCI without FFR (total PCI 60%), three were treated with elective CABG (4%), and 29 conservative (36%). Complete revascularisation was obtained in 84% of the patients who had PCI in the invasive group.
In the conservative group, testing for residual ischaemia was postponed until after discharge. No in-hospital events occurred in this group.
Periprocedural complications
One patient in the invasive group had a coronary dissection caused by the FFR wire, which could be sealed with a stent, resulting in a limited CK rise. One patient had acute vessel closure after FFR measurement but before PCI (FFR < 0.75). After stenting in this patient no reflow occurred, and the patient underwent urgent bypass surgery in poor haemodynamic condition. He died several days later due to heart failure. Two other patients had a non-STEMI after FFR-guided PCI due to side branch occlusion.
Bleeding
Major bleeding, defined as need for transfusion and/or surgical therapy, occurred in six patients, one in the conservative group and five in the invasive group. Three cases were CABG related, two were gastrointestinal bleedings and one was an access-site bleeding related to the primary PCI.
Stent thrombosis
Subacute stent thrombosis (> 24 h) occurred in three patients, all in the invasive group and all related to the initial culprit lesion. There were no cases of late stent thrombosis (> 30 days). Thus, the overall stent thrombosis rate in the study was 2.5% (3/121).
Primary endpoint
Nuclear ejection fraction was assessed at baseline, before any additional intervention, in 91% of the patients (Figure 2).
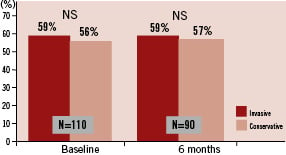
Figure 2. Left ventricular ejection fraction assessed by radionuclide ventriculography in both treatment groups at baseline and after six months (primary endpoint) is depicted. The primary endpoint was available in 90/121 (74%) of the patients. There was no significant difference between the two treatment groups.
EF was 58.9±9.8% in the invasive group versus 55.9±11.2% in the conservative group (p=0.169). After six months, nuclear ejection fraction was again assessed in 74% of the patients. There was no significant difference between the two groups at six months (58.9±9.4% vs. 56.9±9.3%, p=0.362), nor was there a significant change within each group (-0.2±6.7% in the invasive vs. +0.1±7% in the conservative group).
Secondary endpoints
Echocardiography was performed at baseline, before any additional intervention, in 93% of the patients (Table 5).
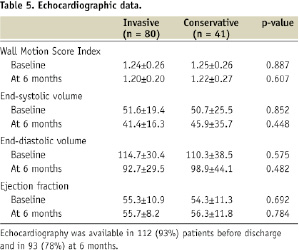
There was no significant difference in EF between the invasive and conservative groups (55.3±10.9% versus 54.3±11.3%, p=0.692) nor in wall motion score index or left ventricular volumes. After six months, echocardiography was repeated in 78% of the patients. End-systolic and end-diastolic volume were reduced in both groups compared to baseline. However, there was no difference in ejection fraction, wall motion score or left ventricular volumes between both groups at six months.
After six months of follow-up, there was no difference in the incidence of major adverse cardiac events between invasively and conservatively treated patients using an intention to treat analysis (21 vs. 22%, p=0.929). Also, there was no difference in non-culprit related MACE (16 vs. 22%, p=0.442). MACE in the conservative group was driven only by PCI of non-culprit lesions after hospital discharge. Of the nine patients who had PCI in this group, four patients developed unstable angina, four had signs of ischaemia during non-invasive testing and one was referred for FFR guided PCI because of stable angina despite adequate medical therapy. Death or MI occurred only in the invasive group (14% vs. 0%, p=0.015, Figure 3). The MI’s were mostly small, in only 4/11 cases (36%) CK-rise was > 3 times the upper limit of normal.
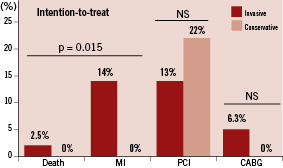
Figure 3. Major cardiac adverse events up to six months of follow-up in both treatment groups (intention-to-treat analysis) are shown. Most events were more frequent in invasively treated patients, except repeat PCI procedures. The difference in the combination of death and myocardial infarction was statistically significant.
A pre-specified per protocol analysis in 105 patients (all 40 patients from the conservative group who were treated conservatively and 65 patients from the invasive group who actually underwent at least one FFR measurement) showed a significant reduction in non-culprit related PCI in the invasive group (6% vs. 22%, P=0.017), but also an increased incidence of death and MI in this group in the per protocol analysis (9% vs. 0%, p=0.079, Figure 4), resulting in a comparable MACE rate in both treatment arms (14 vs. 22%, p=0.295).
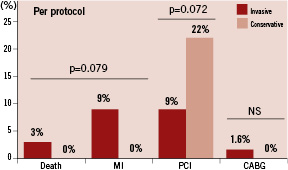
Figure 4. Major cardiac adverse events up to six months of follow-up is depicted in a subgroup of patients that actually was treated according to their randomisation assignment (per protocol analysis in 105 patients). In this analysis, the difference in death and myocardial infarction (Figure 3) was no longer significant. There was a strong trend towards more PCI procedures in the conservative group in this subset. Non-culprit PCI’s were significantly more frequent in the conservative group (22 vs. 6 %, p = 0.017).
Discussion
To our knowledge, this is the first randomised study to investigate the effects of FFR-guided, additional revascularisation early after primary PCI. No beneficial effects of this strategy on left ventricular function could be detected after six months. A reduction in the need for later revascularisation of non-culprit lesions was accomplished, but this was offset by more other cardiac events, mostly myocardial infarctions, resulting in a non-significant difference in total major adverse cardiac events between invasively and conservatively treated patients. An important finding in our study was that 40% of non-culprit lesions did not produce ischaemia.
Effect of an early invasive strategy on LV function
The lack of effect on LV function may be related to the inclusion of a relatively low-risk population with a high ejection fraction at baseline, leaving not much room for further improvement by early additional revascularisation. In a relatively unselected population of patients that underwent primary PCI, including those with single vessel disease, Bolognese et al found that ejection fraction increased from 43% at baseline to 51% after six months16. Average EF in our patients with multivessel disease was already 55% at baseline and did not change significantly at follow-up.
Ejection fraction may not be the optimal parameter to detect small changes in LV function after myocardial infarction. Left ventricular volumes may change considerably without a significant change in ejection fraction and also carry important prognostic information. In this study, although EF was unchanged, both end-systolic and end-diastolic volumes were reduced after six months, but no difference was found between the invasive and conservative groups. Apparently, a delay in (selective) revascularisation in the conservative group did not affect the reduction in left ventricular volumes. This is in line with the finding that multivessel disease is primarily a determinant of late dilatation (after one month)17, whereas early dilatation, if it occurs, is primarily caused by expansion of the infarcted zone18.
Effect of an early invasive strategy on clinical events
In this study early, FFR-guided revascularisation reduced the number of non-culprit related PCI procedures after discharge compared to a conservative strategy. However, it must be noted that the PCI procedures that were prevented were elective, ischaemia-guided procedures in mostly stable patients (6/9), and no deaths or myocardial infarctions could be attributed to these procedures.
Death or myocardial infarction occurred more frequently in the invasive group, driven by more myocardial infarctions. However, the majority of infarctions in this group (6/11) were culprit-related, and occurred before FFR was done, or were related to a new lesion that was not present during primary PCI. The remaining myocardial infarctions (5/80, 6.3%) were related to the invasive strategy. CABG was also more frequent in the invasive group, but should be considered part of the invasive strategy in 3/5 patients, since after follow-up angiography and FFR measurements, PCI was no longer considered the optimal strategy. One death in the invasive arm was related to the early invasive strategy. Bleeding was also more frequent in the invasive group, but was mostly CABG related. All non-CABG related bleeding episodes occurred before repeat angiography in the invasive group.
Although numbers are small, our results suggest that there is an inherent risk of complications attached to an early invasive strategy.
Other studies on additional revascularisation after primary PCI
Only one other study has investigated additional revascularisation after primary PCI in a randomised comparison (HELP-AMI)19. However, in this study additional PCI was performed in the same procedure directly after primary PCI, without ischaemia detection before the intervention. After 12 months of follow-up in 69 randomised patients, repeat interventions were not significantly more frequent in the conservative group, and no benefit in terms of total costs was found. The authors concluded that a staged approach towards multivessel treatment avoids the unnecessary treatment of clinically non-relevant lesions.
Several other studies have also investigated the effects of additional revascularisation, mostly in a non-randomised, retrospective design20-22. Many of these studies conclude that treating multiple lesions during or just after primary PCI is safe and effective. However, selection bias may be an important factor in these studies, and since objective ischaemia was not demonstrated, it is questionable whether treatment of all non-culprit lesions was necessary. In our study, 40% of the non-culprit lesions did not produce ischaemia. It may be quite difficult for even experienced operators to predict which lesions produce ischaemia and which do not23. Moreover, it has been shown that the angiogram that is recorded during acute myocardial infarction is different from angiography performed after several weeks. About 20% of the lesions that are considered significant in the acute phase (> 50%) are no longer significant at a later stage24.
Although the use of FFR has been well validated in patients with previous myocardial infarction25, its results may be less reliable in patients with complex coronary plaques shortly after STEMI. Patients with these kind of lesions were treated with staged PCI and excluded from the study. The optimal treatment strategy in this subpopulation remains to be determined.
Limitations
The study was stopped prematurely because of a slow inclusion rate, resulting in inadequate power to detect a real difference in nuclear EF between the treatment groups after six months. This was further augmented by a relatively high drop-out rate of the primary end-point. However, echocardiographic measurements including ejection fraction and left ventricular volumes showed results that pointed in the same direction, making a clinically relevant effect on left ventricular function unlikely.
In addition, analyses in which missing ejection fractions at six months were modelled from all other available data showed similar results (data not shown).
The population studied was a relatively low-risk population, with a low prevalence of cardiac risk factors and a high baseline EF, possibly as a result of selection bias. The benefit of an aggressive revascularisation strategy may be more pronounced in patients with a clearly reduced EF and a more unfavourable risk profile.
Finally, patients with a chronic occlusion were excluded from the study to make FFR measurements possible. It has been shown that patients with a remaining chronic occlusion after acute myocardial infarction form a subgroup at high risk for new cardiac events26. The treatment strategy in these patients remains to be determined.
Conclusions
In selected patients with relatively preserved left ventricular function, it is unlikely that an invasive strategy towards non-culprit lesions detected during primary angioplasty further improves ejection fraction. The haemodynamic significance of these non-culprit lesions is frequently overestimated (40%). Compared to a conservative strategy, an early ischaemia-guided invasive strategy prevents later PCI procedures but does not result in a reduction of total major adverse cardiac events at six months. These findings support a conservative strategy as currently advocated by the guidelines in these patients.

