
Abstract
Aims: This study independently evaluated the diagnostic performance of electrocardiographic (ECG) criteria to predict the infarct-related artery (IRA) in patients with an acute ST-segment elevation myocardial infarction (STEMI). While a number of ECG criteria have been proposed to predict the IRA in STEMI, many of these “rules” came from modestly sized populations and did not undergo external validation. Therefore, we aimed to evaluate popular criteria from the literature in an independent cohort.
Methods and results: All acute STEMI cases over a 10-year period from a single hospital were retrospectively identified. We excluded patients with a missing pre-intervention ECG, irretrievable angiographic films, prior coronary artery bypass grafting, left bundle branch block, ventricular pacing, or not meeting strict STEMI criteria. After review of the angiograms for the IRA, cases with either no or multiple culprits were excluded. We included 480 subjects meeting STEMI criteria in inferior leads (192, 40%), anterior leads (184, 38%), both anterior and inferior leads (88, 18%), isolated lateral leads (nine, 2%), or a posterior pattern (seven, 1%). Notably, every pattern except isolated lateral STEMI included an IRA in both the right and left coronary arteries.
Conclusions: Existing ECG criteria to predict the IRA in STEMI have modest diagnostic performance when externally validated, and lower than in the original reports. Distinguishing the level of obstruction in the left anterior descending artery remains especially challenging. Hence, their use should be pragmatic when selecting an initial catheter for treating STEMI, since discordances will occur when compared to the actual angiogram.
Abbreviations
D1: first diagonal branch
ECG: electrocardiogram
IRA: infarct-related artery
LAD: left anterior descending
LCA: left coronary artery
LCx: left circumflex
LMCA: left main coronary artery
RCA: right coronary artery
S1: first septal perforator
STD: ST-segment depression
STE: ST-segment elevation
STEMI: ST-segment elevation myocardial infarction
Introduction
The surface electrocardiogram (ECG) provides the cornerstone for diagnosing patients who present with acute chest pain. The subset with ST-segment elevation myocardial infarction (STEMI) proceeds immediately to intravenous thrombolytics or invasive angiography and revascularisation. The ECG in STEMI can denote a medical emergency and has traditionally been used to predict the anatomical location of coronary artery occlusion1.
Based on the anticipated culprit vessel, operators often choose to perform an initial diagnostic angiogram of the contralateral artery. A survey has shown that the majority of interventional cardiologists begin with a diagnostic catheter for the presumed non-culprit artery, then proceed with a guide catheter (58% of responders) or diagnostic catheter (23%) for the culprit vessel2. However, retrospective, observational studies have shown that – logically – the “non-culprit first” approach increases time to reperfusion, albeit by an average of only 13, 4.54, 4 to 65, 66, 77, 88, or 99 minutes. Whether this small increase in ischaemic time translates into worse clinical outcomes has never been explored in a randomised trial, although observational studies suggest no significant impact3-5,7-9. Conversely, in some cases, knowledge of non-culprit anatomy may alter the approach to revascularisation.
Regardless of a “culprit first” or “non-culprit first” approach, a reliable method is required to predict the infarct-related artery (IRA). Several surface ECG criteria have been proposed in previous studies. However, these studies were limited by their modest size and often did not undergo external or extensive validation. Additionally, several of them developed rules only in specific angiographic subgroups, whereas in daily clinical practice an approach based purely on the ECG must be selected. Therefore, our study independently evaluated the performance of ECG criteria to predict the IRA in a large cohort of consecutive patients presenting with STEMI.
Methods
We assembled our cohort from all patients with acute STEMI who presented to a single, tertiary care hospital over a 10-year period (from January 2000 to December 2009) and underwent immediate invasive coronary angiography. The Northwestern Memorial Hospital institutional review board approved this retrospective study. A subset of the cohort examining ECG rules to distinguish anterior STEMI from Takotsubo cardiomyopathy has already been analysed and published10.
Initially, we identified potential subjects by searching the electronic cardiac catheterisation laboratory database for acute infarctions. Each case was reviewed, including surface ECG, angiographic images, and clinical course. Subsequently, we excluded patients with an irretrievable angiogram or ECG, prior coronary artery bypass grafting, absent or multiple culprits, a left bundle branch block or paced ventricular rhythm, under 18 years of age, or those with non-STEMI or unstable angina. A single investigator reviewed the angiogram and clinical course to confirm a true myocardial infarction. In the small number of patients with multiple STEMIs during the decade, only the first episode was included. Hence each unique subject in the cohort had one clear IRA of a native vessel, and its location was recorded locally using the SYNTAX segment11 but also summarised globally as right coronary artery (RCA) versus left coronary artery (LCA) or by the major epicardial vessel. The presence of a bystander total occlusion, felt to be chronic (CTO), was recorded when clear. For the left anterior descending (LAD) artery, we explicitly noted the order of the first septal perforator and first diagonal branch in relation to the occlusion, except in four cases where this information was not recorded. Coronary dominance based on review of angiographic images was recorded as right, left, or codominant, although in clinical practice this information would not be available before angiography. Demographic information and lab values within 24 hours of hospital presentation were extracted when available.
ECG ANALYSIS
From our electronic database, for each subject we retrieved the ECG that was recorded before cardiac catheterisation. If multiple tracings existed, the one with the largest ST-segment deviation was selected. When available, the time of the ECG recording and start time of the coronary angiogram were noted. ECG recordings used 12 standard leads with 10 seconds of data sampled at 250 times/second and 10 mm/mV calibration. These tracings were imported with custom software into MATLAB® (MathWorks, Natick, MA, USA) for off-line analysis. Identification of QRS onset and J-point for each supraventricular beat was performed manually by a single investigator unaware of the angiographic findings. ST-segment deviation was computed as the average difference in millimetres for all marked beats in each lead between QRS onset and subsequent J-point. ST-segment deviations were measured exactly at the J-point, as recommended12. Positive deflections describe ST-segment elevation while negative deviations describe ST-segment depression. The total ST-segment deviation score summed the absolute depression or elevation in all 12 leads.
In accordance with the AHA/ACCF/HRS scientific statement on ECG interpretation in STEMI12, we required that at least two contiguous ECG leads contained significant ST-segment elevation (≥1 mm in all leads except for ≥2 mm in leads V1 to V3). Additionally, we identified isolated posterior STEMI as significant ST depression ≥1 mm in leads V1 and V2. Because we used an average ST-segment deviation from many beats instead of the worst beat on the tracing, as often happens clinically to avoid missing a STEMI, we relaxed the criterion to ≥0.9 mm (or ≥1.9 mm in leads V1 to V3) to reflect real-world practice better. Subjects who did not meet these strict criteria were excluded. Additionally, we performed a sensitivity analysis using the more stringent ≥1 mm (or ≥2 mm in leads V1 to V3) threshold to determine how many subjects would be reclassified.
We divided our cohort into the classic ECG patterns that correlate with anatomical regions: anterior, inferior, posterior, and lateral12 as follows.
– Anterior STEMI: significant ST-segment elevation in two contiguous ECG leads V1 to V6.
– Inferior STEMI: significant ST-segment elevation in two contiguous ECG leads III, aVF, and II.
– Posterior STEMI: no significant ST-segment elevation but significant ST-segment depression in ECG leads V1 and V2.
– Lateral STEMI: significant ST-segment elevation only in ECG leads I and aVL.
Because some ECGs fulfilled criteria for both anterior and inferior STEMI (termed “potentially overlapping”), we subdivided these two groups into five mutually exclusive patterns.
– Pure anterior STEMI: significant ST-segment elevation in two contiguous ECG leads V1 to V6 but no inferior STEMI.
– Pure inferior STEMI: significant ST-segment elevation in two contiguous ECG leads III, aVF, and II but no anterior STEMI.
– Inferoseptal STEMI: significant ST-segment elevation in two contiguous ECG leads V1 to V3 plus two contiguous ECG leads III, aVF, and II.
– Inferolateral STEMI: significant ST-segment elevation in two contiguous ECG leads V4 to V6 plus two contiguous ECG leads III, aVF, and II.
– Mixed anterior and inferior STEMI: not other patterns, but nevertheless significant ST-segment elevation in two contiguous ECG leads V1 to V6 plus two contiguous ECG leads III, aVF, and II.
ECG CRITERIA TO PREDICT STEMI CULPRIT
We began with a major review article describing ECG criteria to predict the IRA in STEMI13. Next, we sought out the original papers14-16 and extracted basic information, including ECG criteria, sample size, sensitivity, and specificity. For some rules, we were able to find subsequent cohorts that had been evaluated independently17,18. We then applied these ECG criteria to our own cohort.
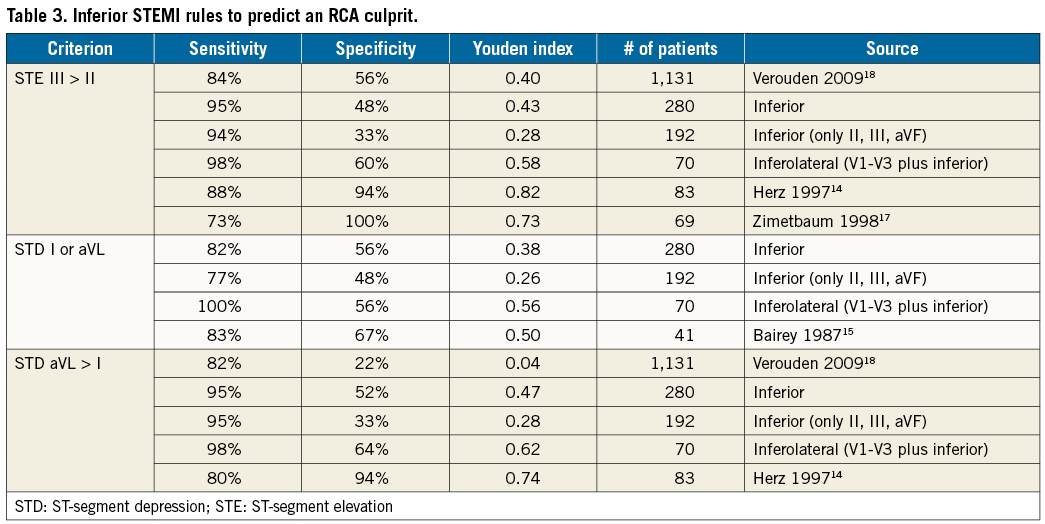
– Inferior STEMI: ST-segment elevation in lead III greater than lead II indicates an RCA culprit14.
Inferior STEMI: ST-segment depression in lead I or aVL ≥1 mm indicates an RCA culprit15.
Inferior STEMI: ST-segment depression in aVL greater than lead I indicates an RCA culprit14.
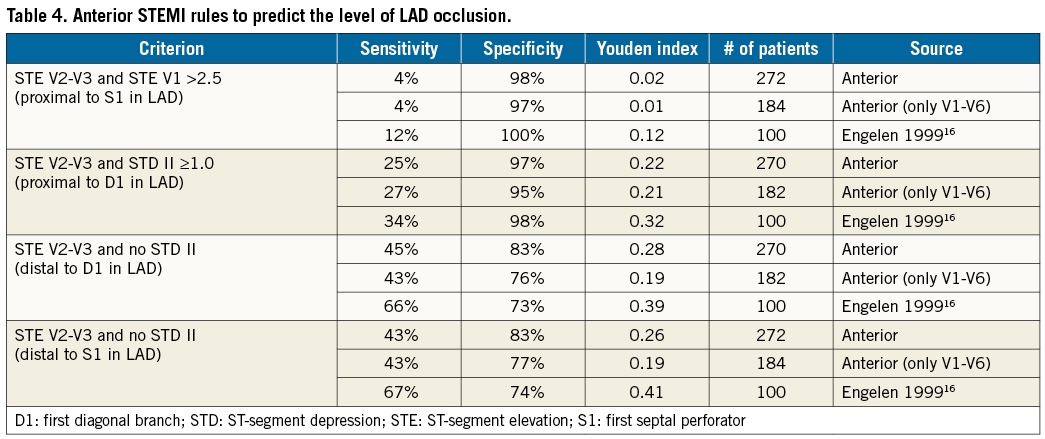
– Anterior STEMI: ST-segment elevation in leads V2 and V3 ≥2 mm plus a variety of added criteria to indicate location along the LAD16 when proximal to the first septal perforator if ST-segment elevation >2.5 mm in lead V1; distal to the first septal perforator if no ST-segment depression in lead II; proximal to the first diagonal branch if ST-segment depression ≥1 mm in lead II; and distal to the first diagonal branch if no ST-segment depression in lead II.
STATISTICAL ANALYSIS
Analysis was performed using R, Version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). We employed standard statistical techniques. To summarise diagnostic performance, we computed the Youden index, equal to the sum of sensitivity plus specificity minus 119. A gold standard test has a Youden index of 1.0, while a useless test has a Youden index of zero. For each ECG pattern, we calculated the ratio of RCA to LCA culprits. A value >1 reflects a higher probability for an IRA in the RCA, while a value <1 reflects a higher probability for the LCA. Applicable tests were two-tailed, and p<0.05 was considered statistically significant.
Results
Over a 10-year period we examined 882 patients in our electronic database, of whom 480 met STEMI inclusion criteria as detailed in Figure 1. Demographic and clinical characteristics can be found in Table 1 and reflect a typical infarct population. Total ST-segment deviations were always >5 mm except for two cases (4.6 and 4.8 mm), with a median value of 14 mm (interquartile range 10 to 21 mm). Valid time stamps for both ECG and angiogram were available in 392 (81.7%) cases, of which 132 (33.7%) were within 30 minutes of the angiogram and a further 96 (24.5%) within 60 minutes. A total of 23 subjects (5% of the included cohort) would not have been classified as STEMI using ≥1 mm (or ≥2 mm in leads V1 to V3) instead of 0.1 mm less, including 14 anterior (10 LAD culprits, two left circumflex [LCx], two RCA), eight inferior (six RCA culprits, one LCx, one LAD), and one lateral (LCx culprit) patterns.
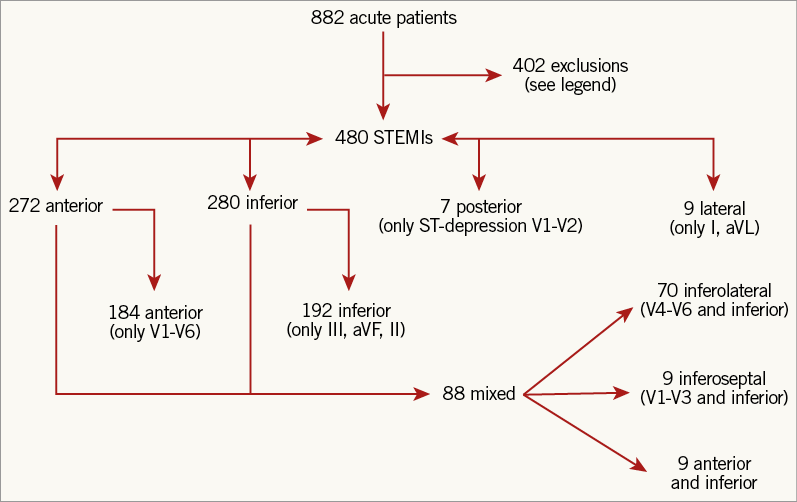
Figure 1. Study flow chart. Starting with the total number of acute infarction cases over 10 years, we excluded patients who did not meet criteria for STEMI with an available ECG and angiogram. Exclusions: 247 did not meet STEMI criteria; 51 had prior CABG; 41 had multiple culprits or no culprit; 20 with duplicate event; 17 without retrievable ECG; 16 had LBBB or paced ventricular rhythm; 9 without retrievable angiogram; and 1 under the age of 18 years old. Included patients had various ECG patterns of ST-segment elevation, as further detailed in Table 2.
The vast majority of the cohort had some type of anterior or inferior STEMI (96%), while less than 5% had an isolated lateral or posterior STEMI. Table 2 provides the distribution of culprit vessels for each ECG pattern among the left main coronary artery (LMCA), LAD, LCx, and RCA. As in Figure 1, patterns in Table 2 are separated between those that potentially overlap versus the mutually exclusive grouping. Notably, all patterns except lateral STEMI could result from either an LCA or RCA culprit, making an absolute rule based solely on ST-segment elevation impossible. For the 12 cases with a clear, chronic total occlusion, only one case of an acute LAD culprit produced an isolated, inferior STEMI (no significant anterior ST-segment elevation) with chronic RCA occlusion; all other chronic total occlusions displayed agreement between acute culprit vessel and ECG pattern (anterior with LAD culprit; inferior or posterior with RCA or LCx culprit). As specific examples of discordance, Figure 2 shows an inferior STEMI due to an LCx culprit (despite ST-segment elevation in lead III larger than lead II combined with ST-segment depression in leads I and aVL); Figure 3 shows an anterior STEMI due to an RCA culprit.
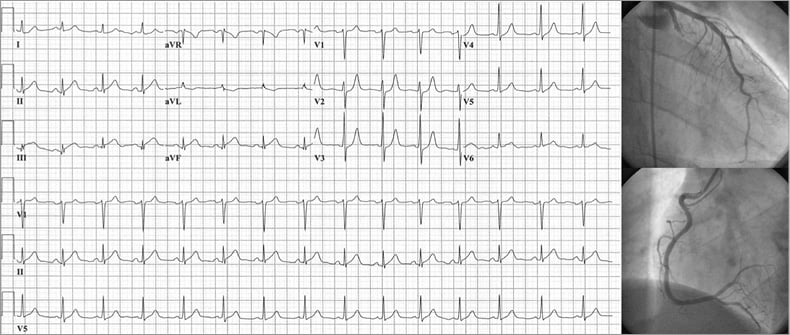
Figure 2. Example of inferior STEMI. ECG and corresponding coronary angiogram depict an inferior STEMI with ST-segment elevation in lead III >lead II and <1 mm ST-segment depression in leads I and aVL, where the infarct-related artery was nevertheless the proximal left circumflex.
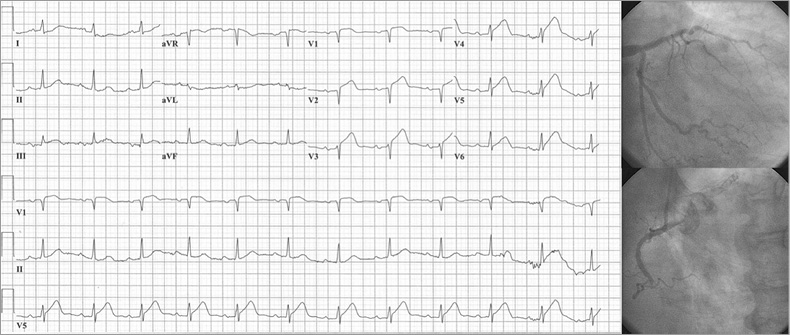
Figure 3. Example of anterior STEMI. ECG and corresponding coronary angiogram depict an anterior STEMI where the infarct-related artery was the mid RCA despite a complete lack of significant inferior ST-segment deviation.
PREDICTING THE CULPRIT FOR INFERIOR STEMI
A total of 280 subjects had an inferior STEMI, with an almost 3-to-1 dominance of RCA culprits (Table 2). If only the inferior leads were involved, then the ratio climbed to almost 5-to-1. Proposed rules for separating RCA from LCA culprits had modest diagnostic performance, as summarised in Table 3, and always worse in validation cohorts than the initial derivation cohort. However, the Youden index improved when moving from isolated inferior STEMI to our entire cohort of inferior STEMI to inferolateral STEMI, probably due to the decreasing likelihood of an RCA culprit, as seen in Table 2 by the RCA/LCA ratio.
PREDICTING THE CULPRIT FOR ANTERIOR STEMI
A total of 272 subjects had an anterior STEMI, with an approximate 5-to-1 dominance of LCA culprits (Table 2). If only the anterior leads were involved, then the ratio climbed to over 30-to-1. In either case, culprits were found in the RCA, implying that anterior STEMI does not always arise from the LCA. As summarised in Table 4, proposed rules for distinguishing the site of LAD occlusion in isolated anterior STEMI performed very poorly in our validation cohort and uniformly worse than in the derivation cohort. For predicting an occlusion proximal to the first septal perforator, the Youden index was near zero, implying an almost worthless diagnostic test.
Discussion
Predicting the culprit vessel in STEMI carries clinical relevance for several reasons. Most importantly, interventional cardiologists would like to know the IRA in advance of the procedure to choose either a “culprit first” or a “non-culprit first” approach2. Although neither strategy has been tested in a randomised trial of outcomes, the correct choice can clearly make a difference for select patients who present in extremis such as with cardiogenic shock, complete heart block, or electrical storm. Additionally, understanding the culprit artery allows anticipation of clinical scenarios such as conduction disturbances and mechanical complications.
Our cohort provides a large, independent validation of commonly used criteria for predicting the IRA in STEMI. We found that either the RCA or LCA can produce every ECG pattern with the exception of isolated lateral STEMI. Therefore, using only the location of ST-segment elevation cannot serve as a foolproof rule. Furthermore, many proposed criteria have poor diagnostic performance when validated outside their derivation cohorts, suggesting a lack of generalisability. As such, their successful application to clinical practice will remain limited. Indeed, the pre-test probabilities in our Table 2 probably provide a more realistic guide for the IRA.
A review of prior work highlights an important difference with our study. Some previous cohorts were first selected on the basis of angiographic criteria before studying the ECG patterns. For example, one study began by searching for RCA or LCx culprits in STEMI, then applying inferior ECG criteria, thereby explicitly excluding LAD culprits18. By design, such a search strategy differs fundamentally from clinical practice where the ECG comes first and must predict the invasive IRA. Our Table 2 demonstrates that 8% of inferior STEMI arise from the LAD.
For inferior or inferolateral STEMI, prediction rules cannot anticipate coronary dominance. As shown in Table 2, an almost 2-to-1 split exists between RCA and LCA culprits when significant ST-segment elevations occur in both inferior leads and V4 to V6 (an inferolateral STEMI). This ratio approximates the population prevalence of right- versus left-dominant circulations, typically 80% pure right dominant20, although the dominant vessel may not always be the culprit. Thus, while reasonable in general, we cannot expect an ECG to provide us with information regarding dominance at the level of a single patient. Similarly, the presence of unanticipated chronic total occlusions or “wrap around” LAD or posterior descending arteries cannot be known from the ECG, but can affect the resulting ECG pattern. Therefore, the modest and imperfect predictive ability of rules for STEMI culprits should not be surprising when considering anatomic variations.
For the level of LAD occlusion in anterior STEMI, our results show that proposed rules have very little diagnostic value. While disappointing, the practical consequences for identifying the LCA as the culprit remain minimal, as the vast majority of (but not all) anterior STEMI arises from the LCA and therefore will not affect the choice of vessel order during an invasive procedure.
The modest diagnostic performances in Table 3 and Table 4 serve as an additional warning regarding overenthusiastic adoption of results without independent confirmation. Our sample size for validation was larger than any of the original derivation cohorts, again providing thoughtful restraint and shaping realistic expectations for results from small populations. For this reason, we did not seek to derive new rules in our cohort, but instead focused on the more important task of independently examining existing criteria.
Logical future work that builds on our results would be a randomised trial comparing “culprit first” to “non-culprit first” interventional approaches in STEMI. While a topic of frequent debate in the literature21-23, the practical consequence for ordering the sequence of angiography and intervention in STEMI has not been definitively established. Both methods are used routinely in practice, STEMI occurs frequently, and the general idea falls under the umbrella of comparative effectiveness research wherein informed consent may be simplified greatly24. In the protocol of such a trial, our Table 2 could serve to develop rules for how to select the culprit, with full awareness of real-world variation and hence modest diagnostic performance regardless of the chosen strategy.
Limitations
As our data were collected retrospectively, our results share all the well-known limitations of this design. We did not examine lead V4R due to its inconsistent recording, although that lead might be useful in distinguishing between the RCA and LCx in inferior STEMI1. We excluded patients with prior bypass grafting as their more complex anatomy can produce unusual ECG patterns during STEMI. However, post-bypass patients remain a small minority of STEMI populations25 and their history is almost always available before beginning cardiac catheterisation. Following current recommendations12, we measured ST-segment deviation exactly at the J-point, in contrast to some – but not all16 – earlier publications that added an 80-millisecond delay14,15,17, leading to small differences in definitions. While our strict STEMI criteria come from the guidelines12, in practice a borderline ECG in the correct clinical setting will prompt immediate angiography. Although we excluded these tracings, apart from a small 5% minority with elevations within 0.1 mm of the criteria, probably the results would have been even less predictive for the IRA due to smaller ST-segment deviations. The vast majority, but not all, of the cohort had a total ST-segment deviation >5 mm, previously found to be highly predictive of the culprit artery in contrast to smaller amounts of ST-segment deviation26. The time between the ECG and angiogram was not uniformly recorded or standardised; a 12-lead ECG at the time of angiography would have provided simultaneous assessment of anatomy and electrocardiography, although not reflective of routine clinical practice. We did not systematically record if a “wrap around” LAD supplied the inferior region, which might explain some cases of inferior STEMI due to a LAD culprit, although clinically this information would not be available prior to angiography. Finally, we applied single rules in isolation and did not combine existing rules; potentially a combination might improve diagnostic performance if the incorrect results from one rule were corrected by a second rule.
Conclusions
Existing ECG criteria to predict the IRA in STEMI have modest diagnostic performance when externally validated, and lower overall than in the original reports. Distinguishing the level of obstruction in the LAD artery remains especially challenging. Hence, use of ECG prediction rules should be pragmatic when selecting an initial catheter for treating STEMI, since discordances will occur when compared to the actual anatomy.
| Impact on daily practice Existing electrocardiographic (ECG) criteria to predict the infarct-related artery in ST-segment elevation myocardial infarction (STEMI) have modest diagnostic performance when externally validated, and lower overall than in the original reports. Distinguishing the level of obstruction in the LAD artery remains especially challenging. Hence use of ECG prediction rules should be pragmatic when selecting an initial catheter for treating STEMI, since discordances will occur when compared to the actual anatomy. |
Conflict of interest statement
J. Flaherty has received investigator and proctor fees from Direct Flow Medical (SALUS, NCT01932099) for a device study in aortic stenosis. L. Dekker has received consultancy fees from Medtronic and St. Jude Medical, and research support from Medtronic, St. Jude Medical, and Philips. N. Johnson has received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis, has an institutional licensing and consulting agreement with Boston Scientific for the smart minimum FFR algorithm, and has received significant institutional research support from St. Jude Medical/Abbott (CONTRAST, NCT02184117) and Volcano/Philips Corporation (DEFINE-FLOW, NCT02328820) for studies involving intracoronary pressure and flow sensors. The other authors have no conflicts of interest to declare.

