The “Will this trial change my practice?” sessions at PCR
The aim of the article is to capture the session at EuroPCR 2015, communicate the analysis of the trialists, and report the views expressed in the interactive discussion.
Abbreviations
CI: confidence interval
HR: hazard ratio
ESC: European Society of Cardiology
GPIIb/IIIa: glycoprotein IIb/IIIa receptor antagonists
NNH: number needed to harm
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction to the session
Controversy persists over the use of thrombus aspiration in ST-elevation myocardial infarction (STEMI). Despite differing results from clinical trials and diminishing evidence for the clinical benefit of thrombus aspiration, a significant proportion of interventional cardiologists continue to use thrombus aspiration either in all cases or selectively on the basis of a proposed mechanism of action that seems intuitive. In the light of the recent TOTAL trial, this important topic was discussed this year at EuroPCR 2015 in a “Will this trial change my practice?” session.
The chairs (Andreas Baumbach and Stefan James) led an expert panel through a review of the clinical implications of the TOTAL trial and discussed the relevance of the results in everyday practice. In an initial poll of attendees, approximately 40% of the audience still performed selective manual aspiration in STEMI cases but fewer than 10% reported performing thrombus aspiration routinely in all STEMI cases. Most felt they would continue to use aspiration in certain circumstances including patients with prolonged pain to balloon times, TIMI 0-1 flow at presentation and high thrombus burden on initial angiography.
The case presentation
Michael Haude presented a complex patient presenting with an inferior STEMI with recurrent stent thrombosis of the right coronary artery (RCA) previously treated with five bare metal stents. The RCA was occluded at the ostium with TIMI 0 flow. The questions to be discussed were how best to treat the patient and whether the evidence supports using thrombus aspiration in such a patient with a large thrombus burden.
The routine aspiration thrombectomy with percutaneous coronary intervention versus PCI alone in patients with ST-elevation myocardial infarction undergoing primary PCI (TOTAL) trial was a multicentre, prospective, randomised, controlled, open-label trial comparing routine upfront manual thrombus aspiration followed by PCI to PCI alone (only bail-out thrombus aspiration allowed) in patients with STEMI1,2. The primary endpoint was a composite of cardiovascular death, myocardial infarction (MI), cardiogenic shock and Class IV New York Heart Association (NYHA) heart failure at 180 days. A total of 10,732 patients presenting with STEMI within 12 hours of the onset of chest pain were enrolled in the trial. TOTAL found no significant difference in the primary endpoint (6.9% in the aspiration group versus 7.0% in the no aspiration group, hazard ratio [HR] 0.99, 95% CI: 0.85-1.15). There was a significant increase in the rate of stroke in the aspiration group at both 30 and 180 days (0.7% versus 0.3%, HR 2.06, 95% CI: 1.13-3.75 and 1.0% versus 0.5%, HR 2.08, 95% CI: 1.29-3.35, respectively).
Background: what was known before the trial?
Keith Oldroyd reviewed what was known about thrombus aspiration before TOTAL. The TAPAS trial was a single-centre trial of 1,071 patients, randomised to either aspiration or PCI alone. TAPAS demonstrated improved post-procedural myocardial blush score, and improved cardiac mortality and reduced non-fatal myocardial infarction at one year3. As a result of the TAPAS trial findings, the 2012 ESC STEMI guidelines concluded that manual thrombus aspiration was reasonable for patients undergoing primary PCI (class IIa, level of evidence B)4. The Thrombus Aspiration in ST-Elevation myocardial infarction in Scandinavia (TASTE) multicentre trial randomised 7,244 STEMI patients from the national Swedish Coronary Angiography and Angioplasty Registry (SCAAR) and demonstrated no difference in all-cause mortality with aspiration at either 30 days or one year5,6. Following TASTE, the 2014 ESC guidelines for myocardial revascularisation stated that thrombus aspiration may be used in selected patients (a downgrade to class IIb, level of evidence B)7.
Trial analysis: summary of the trialists’ critical review
Alexandra Lansky reviewed the TOTAL trial design and outcomes. Before the trial was initiated, the investigators performed a unique online survey of 477 participants in order to plan the trial, and to establish current use of aspiration in STEMI and the perceived associated complications2. This allowed the investigators to design the trial to meet the needs and expectations of the cardiology community. In Alexandra Lansky’s opinion, TOTAL was the first and only properly powered trial of thrombus aspiration. It represented contemporary practice (recruitment between 2010 and 2014), with broad inclusion and minimal exclusion criteria, and 48% of patients were recruited from European centres. The TOTAL trial demonstrated no differences in the composite primary endpoint (Figure 1), and no significant differences between any of its components. Furthermore, there were no demonstrable benefits in any of the pre-specified subgroups including baseline TIMI flow 0-1, anterior vs. non-anterior MI, large and very large thrombus burden or longer times from onset of pain consistent with the TASTE trial.
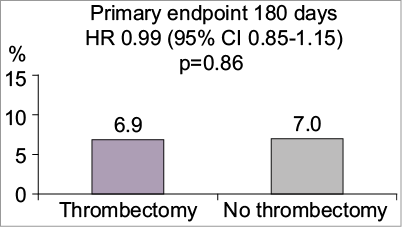
Figure 1. Primary composite cardiovascular death, re-MI, cardiogenic shock or NYHA class IV heart failure within 180 days. Stroke within 30 days: Thrombectomy 7% vs no thrombectomy 0.3% p=0.02. No significant difference in cardiovascular death, re-MI or target-vessel revascularisation. Figure reproduced from the PCR Trials Book (© 2015 Europa Digital & Publishing)
Aspiration was associated with approximately a doubling of the stroke rate at both 30 days and 180 days, with continued separation of the Kaplan-Meier curves and greater accrual of strokes in the thrombus aspiration group compared to controls from 30 days to six months. The reason for this is not obvious: the threefold higher risk in acute stroke rates seen as early as 48 hours (p=0.025) (S. Jolly, presented Hotline session EuroPCR 2015) can be plausibly attributed to mechanical factors associated with thrombus aspiration at the time of the procedure; however, the longer-term increase was not easy to explain. As the trial was not blinded, it is possible that differences in antiplatelet selection or pharmacology between treatment groups might explain the 19 additional strokes in the TA group compared to only nine in the PCI alone group between 30 days and six months. To put this into context, the absolute increase in stroke rate at 30 days corresponds to one stroke for every 250 patients treated with thrombectomy (number needed to harm [NNH]=250).
There were limitations to the trial: the operators were not blinded to the treatment delivered which could have introduced bias, fewer GPIIb/IIIa antagonists were used in the aspiration arm and 8.5% of the PCI alone patients crossed over to receive aspiration. Nevertheless, Alexandra Lansky felt TOTAL was the only powered and definitive trial of thrombus aspiration in myocardial infarction, emphasising that to demonstrate cardiac mortality benefit in the TAPAS and TASTE trials would have required close to 20,000 patients and thus both studies were inadequately powered. TASTE had shown that there was no clinical benefit from aspiration, whilst TOTAL had gone further confirming that aspiration does not provide benefit and indeed appears to cause harm.
The case resolution and the practitioners’ view
Michael Haude then presented the conclusion to his case, demonstrating apparently successful use of thrombus aspiration. However, although post-aspiration angiography showed no thrombus, OCT showed that a large thrombus burden remained. A good result was eventually achieved with a mesh-covered stent. M. Haude stated that, in his view, if aspiration is performed correctly and carefully it is a useful technique whose benefits include removal of vasoactive substances in addition to thrombus and the option of local drug delivery.
Final audience poll
At the end of the session, Andreas Baumbach and Stefan James re-polled the audience. No-one would now perform routine aspiration, with less than 20% of the audience continuing to use aspiration even in selective cases. The feeling of the audience was that TOTAL has significantly changed their clinical practice.
| Summary box Arguments for a change in practice: – Two large multicentre trials (TASTE and TOTAL) have now shown no clinical benefit of aspiration in the routine treatment of STEMI – TOTAL showed that aspiration was associated with harm (an increased stroke rate at 30 and 180 days) Arguments against a change in practice: – A smaller single-centre trial (TAPAS) showed a mortality benefit with aspiration in STEMI – The proposed mechanism of action seems intuitive |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Session format
Chairperson: A. Baumbach
Co-chairperson: S. James
Participant: M. Behan
Introduction: the trial headlines. - A. Baumbach
A case: how should I treat? - M. Haude
Discussion: what is common practice?
What was known before TOTAL. - K.G. Oldroyd
Trialists’ review: methods, results, conclusion. - A. Lansky
Discussion and audience interaction.
Case conclusion: how does this apply to my practice? M. Haude
Discussion and audience interaction.
Session evaluation and key lessons. - S. James

