
Abstract
Aims: It is unclear whether microvascular dysfunction following ST-elevation myocardial infarction (STEMI) is prognostic for long-term left ventricular function (LVF), and whether recovery of the microvasculature status is associated with LVF improvement. The aim of this study was to assess whether microvascular dysfunction in the infarct-related artery (IRA), as assessed by coronary flow reserve (CFR) within one week after PPCI, was associated with LVF at both four months and two years.
Methods and results: In 62 patients, CFR and hyperaemic microvascular resistance index (HMRI) in the IRA were assessed by intracoronary Doppler flow measurements within one week and at four months. CMR was performed at the same time points and also at two years. CFR at baseline was associated with left ventricular ejection fraction (LVEF) at four months (β=4.66, SE=2.10; p=0.03) and at two-year follow-up (β=5.84, SE=2.45; p=0.02). HMRI was not associated with LVF. In large infarcts, absolute improvement of CFR in the first four months was associated with LVEF improvement (β=5.09, SE=1.86, p=0.01).
Conclusions: Microvascular dysfunction, assessed by CFR, in the subacute phase of STEMI is prognostic for LVEF at four months and two years. This underlines the pivotal role of microvascular dysfunction following STEMI.
Abbreviations
APV: average peak velocity
BMI: body mass index
BMRI: baseline microvascular resistance index
CFR: coronary flow reserve
CK-MB: creatine kinase myocardial band
CMR: cardiac magnetic resonance
HMRI: hyperaemic microvascular resistance index
IRA: infarct-related artery
IS: infarct size
LV: left ventricle
LVEDV: left ventricular end-diastolic volume
LVEF: left ventricular ejection fraction
LVESV: left ventricular end-systolic volume
LVF: left ventricular function
MVO: microvascular obstruction
PPCI: primary percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
In 30 to 40% of ST-elevation myocardial infarction (STEMI) patients, myocardial tissue perfusion remains compromised, despite restoration of epicardial patency after primary percutaneous coronary intervention (PPCI)1. Microvascular dysfunction, due to reperfusion injury, endothelial dysfunction, neurohumoral activation or intramyocardial haemorrhage, has been described as a possible cause for this phenomenon2-4. This is, in turn, proposed as the pathophysiological mechanism in the development of adverse left ventricular (LV) remodelling and overt heart failure1,5,6.
Microvascular function can be measured invasively with coronary flow reserve (CFR) and hyperaemic microvascular resistance index (HMRI). CFR estimates the vasodilatory capacity of the coronary microvascular bed7. Insights into the temporal evolution of microvascular dysfunction and its implications on long-term left ventricular function (LVF) are currently lacking. The primary objective of this study was to assess whether microvascular dysfunction in the infarct-related artery (IRA), as assessed by CFR within one week after PPCI, was associated with LVF at two years as assessed by cardiac magnetic resonance (CMR) imaging. This was compared to the prognostic value of HMRI. Secondary objectives were to assess whether an improvement in microvascular function was associated with LVF improvement.
Methods
STUDY POPULATION AND PROCEDURES
For the current study, we included a subpopulation of the HEBE trial, designed to assess the effect of bone marrow mononuclear cell therapy on cardiac improvement in STEMI patients8-10. This substudy included patients who underwent paired intracoronary flow measurements in the Academic Medical Center and the VU University Medical Center, Amsterdam (n=64). Patients who experienced a reinfarction during follow-up (n=2) were excluded. The study was conducted in accordance with the Declaration of Helsinki and the study protocol was approved by the institutional review boards of both centres.
INTRACORONARY DOPPLER FLOW MEASUREMENTS AND DATA ANALYSIS
A bolus of 0.1 mg nitroglycerine was administered intracoronarily prior to Doppler flow measurements. Intracoronary flow was measured with a 0.014-inch Doppler-tipped guidewire (FloWire®; Volcano Corporation, San Diego, CA, USA) that was positioned distal to the previously implanted stent in the IRA. Following optimisation of the Doppler signal, the average peak flow velocity recordings were obtained before and after induction of hyperaemia by an intracoronary bolus administration of 20 to 40 μg adenosine. Also, intracoronary flow was assessed in the non-IRA (reference artery), if there was <30% stenosis. The position of the Doppler-tipped guidewire in both arteries was documented on angiography at baseline in order to obtain a similar guidewire position at four months.
Doppler flow velocity was recorded continuously and analysed offline by an independent investigator10. The following parameters were assessed: systolic and diastolic mean aortic pressure and average peak flow velocity at baseline and hyperaemia. CFR was determined as the ratio of hyperaemic to baseline average peak flow velocity. An impaired CFR was defined at the cut-off value of <2.0, according to the mean of the current study population. The relative CFR was calculated as the absolute CFR in the IRA divided by the absolute CFR in the reference vessel. HMRI was calculated by dividing the mean aortic pressure by the average peak flow velocity during maximum hyperaemia. Improvement of microvascular function could be assessed by calculating the difference (∆) between CFR and HMRI at four months and baseline. Both the absolute (∆) and relative (%) differences of both indices were assessed.
CARDIAC MAGNETIC RESONANCE AND DATA ANALYSIS
CMR was performed on a clinical MRI scanner at 4±2 days following PPCI (baseline) and at four and 24 months, as previously described8. In short, both cine and delayed contrast-enhanced CMR was performed to measure LVF, infarct size, transmurality and the presence of microvascular obstruction. The changes (∆) in left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) index were evaluated as the absolute and percentage increases or decreases from baseline to four months and baseline to two years. Total infarct size was determined as previously described using a predefined and standardised definition of hyperenhancement, expressed as a percentage of LV mass8. Transmurality was determined by dividing the hyperenhanced area by the total area of the predefined segment. MVO was defined as any region of hypoenhancement within the hyperenhanced infarcted area8. CMR data were analysed in a core lab using a dedicated software package (Mass 2008 beta; Medis, Leiden, the Netherlands), blinded to the intracoronary Doppler flow measurements.
STATISTICAL ANALYSIS
Normally distributed data are expressed as mean±SD and for non-normally distributed data the median value (25th to 75th percentile) is provided. Categorical variables are presented as number (%) and compared by the chi-square test. A Student’s t-test or a one-way analysis of variance was used to compare data with a normal distribution of continuous variables and a Kruskal-Wallis test for non-normally distributed continuous variables.
A univariate linear regression model was used to assess whether CFR and HMRI measured in the subacute phase were associated with global left ventricular volumes and function at baseline, four months and two years.
In a subsequent multivariate linear regression model, the prognostic value of CFR in the subacute phase was assessed with other known patient and angiographic characteristics associated with LVEF (%) at two years11,12. Based on the cut-off value of CFR <2, patients were classified into those with an impaired (CFR <2) or preserved microvascular function (CFR ≥2) at baseline. LVEF, LVEDV and LVESV index measured at baseline, four months and at two years were compared in both groups. The temporal change in LVEF, LVEDV and LVESV index was also compared between the groups.
Whether improvement in CFR and HMRI within the initial four months was concurrently associated with improvement in LVEF was investigated for two different infarct size groups stratified according to the median infarct size.
A p-value <0.05 was considered statistically significant. All statistical analysis was performed using SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
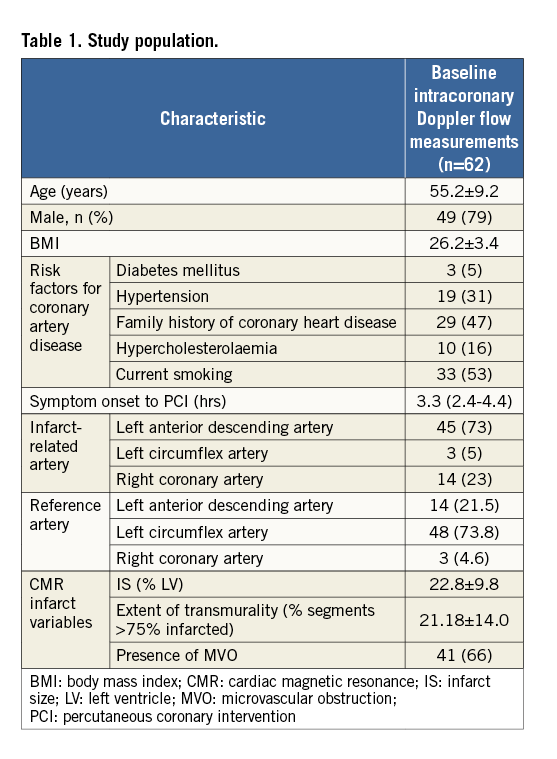
The study population is described in Table 1. Mean age was 55±9 years and the left anterior descending artery (73%) was most frequently the IRA. Baseline characteristics of patients who underwent baseline intracoronary measurements did not differ from those who underwent paired measurements.
RELATION BETWEEN CORONARY MICROVASCULAR FUNCTION AND LEFT VENTRICULAR FUNCTION
During initial coronary flow measurements, CFR in the IRA was significantly lower compared to the reference vessel (2.03±0.53 vs. 2.7±0.5; p<0.001). The observed decreased CFR in the IRA was the result of an increased baseline and decreased hyperaemic average peak velocity (Table 2).
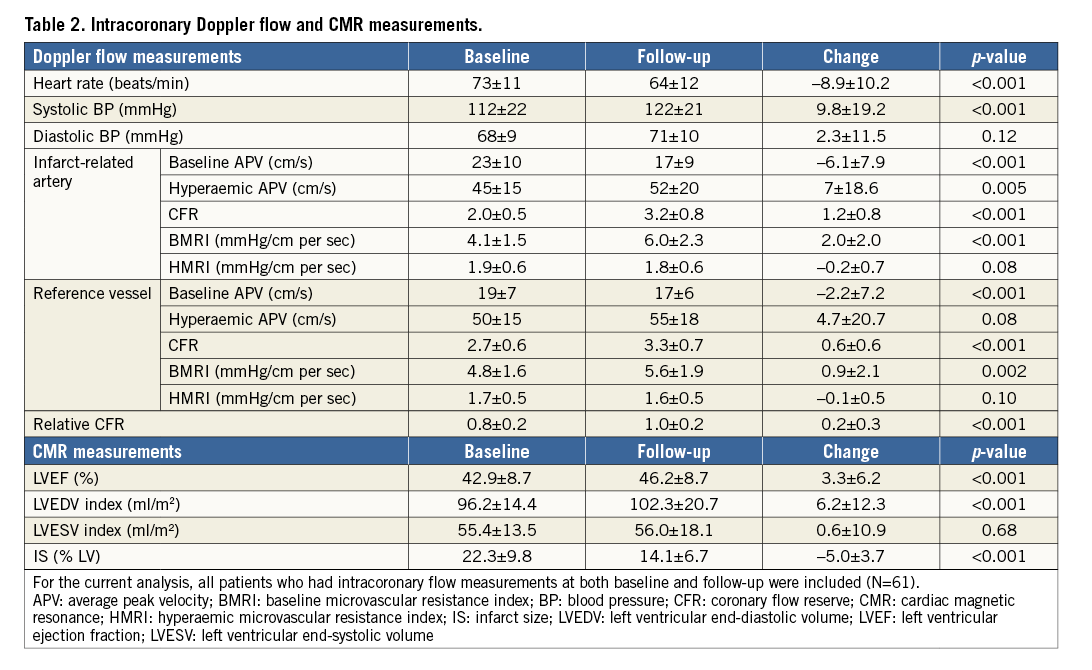
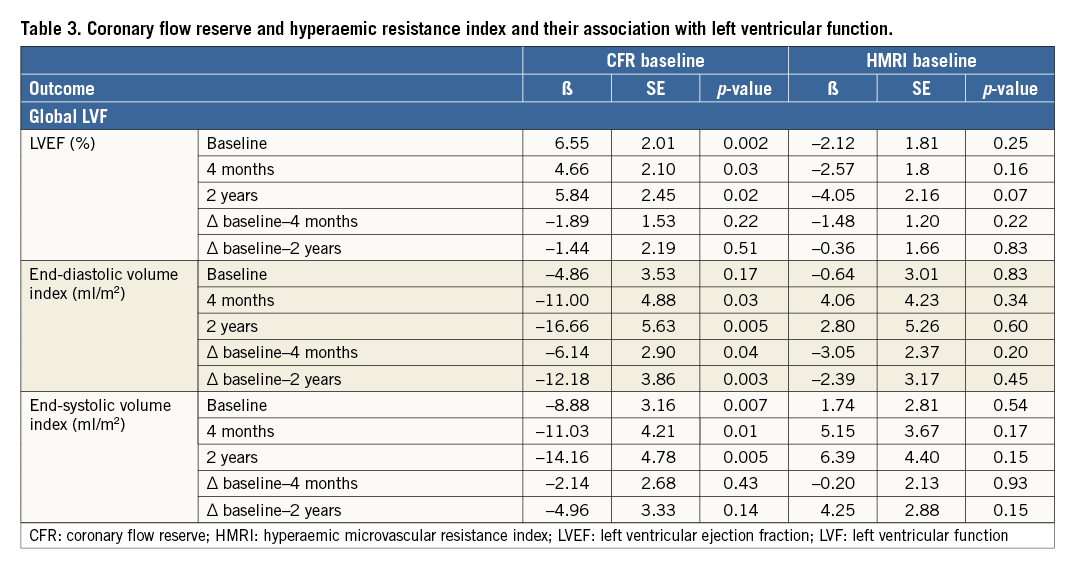
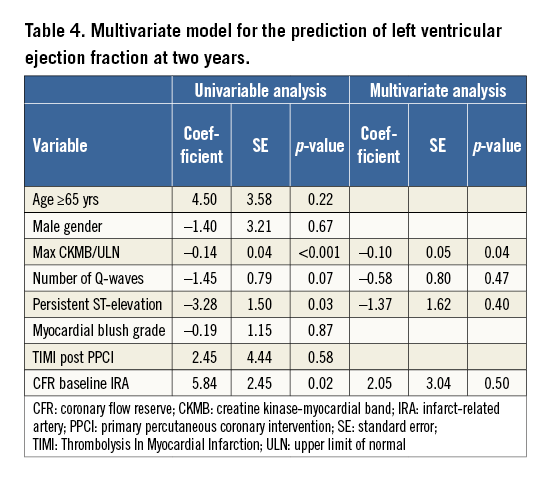
CFR in the subacute phase was associated with LVEF at four months (β=4.66, SE=2.10; p=0.03) and at two-year follow-up (β=5.84, SE=2.45; p=0.02) and inversely associated with LVEDV and LVESV index at four months and two years (Table 3). Additionally, CFR in the subacute phase was associated with the changes in LVEDV. HMRI in the subacute phase was not associated with LVF. In a multivariate regression model, CFR was not associated with LVEF at two years (Table 4).
IMPAIRED MICROVASCULAR FUNCTION AND LEFT VENTRICULAR FUNCTION
An impaired coronary microvascular function (CFR <2) in the subacute phase was observed in 36 patients (58%) with a mean CFR of 1.67±0.19. These patients had a larger infarct size than patients with a CFR >2 (25.3±7.7% LV vs. 19.5±11.3% LV; p=0.04).
As shown in Figure 1, patients with a CFR <2 had a lower LVEF at baseline (41.0±8.2% vs. 45.6±8.8%, p=0.04) and at two years (46.1±8.8% vs. 54.3±8.1%, p<0.001). Patients with a CFR <2 experienced a larger increase in LVEDV and LVESV between baseline and two years in comparison to patients with a CFR ≥2, respectively 8.5±15.1 ml/m2 vs. –1.5±13.9 ml/m2 (p=0.02) and 0.71±12.5 ml/m2 vs. –6.7±11.0 ml/m2 (p=0.03).
CHANGE IN CORONARY MICROVASCULAR FUNCTION AND LEFT VENTRICULAR FUNCTION
Changes in CFR and HMRI were investigated in two groups, stratified to median infarct size (24.2% LV) at baseline. Patients with large infarct sizes (>median infarct size) had a lower CFR at baseline. However, these patients had more improvement in CFR within the initial four months compared to patients with smaller infarct sizes (Table 5). In these patients, absolute CFR improvement was concordantly associated with LVEF improvement within four months (β=5.09, SE=1.86, p=0.01) (Figure 1).
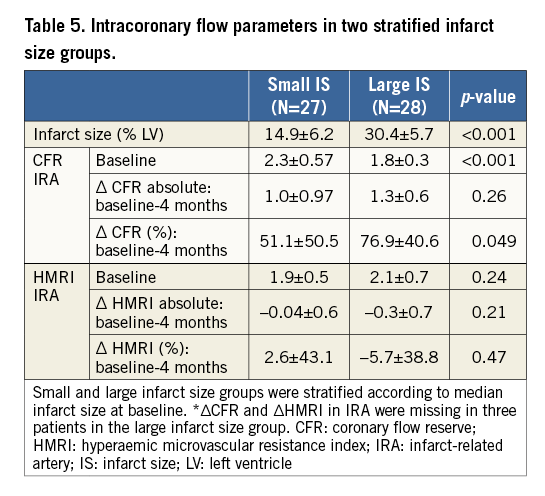
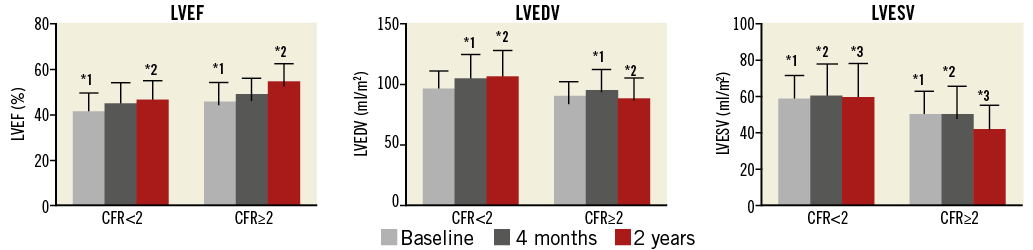
Figure 1. Left ventricular function in patients with initially impaired and normal coronary flow reserve. Bar graph on the left ventricular function parameters in patients with impaired and normal coronary flow reserve at baseline, defined according to the cut-off value CFR <2. The evolution of the left ventricular function parameters over baseline, four months and two years is visualised in both groups. The * indicates a p<0.05 and the number indicates the two matching groups corresponding to the p-value. CFR: coronary flow reserve; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume
In patients with a smaller infarct size, absolute CFR improvement was not associated with LVEF improvement between baseline and four months (β=–0.30, SE=1.24, p=0.81) (Figure 2). When considering HMRI, a trend was observed between the absolute decrease in HMRI within the initial four months and LVEF improvement (β=–3.38, SE=1.67, p=0.054). Similarly, patients with a greater decrease in microvascular resistance (<∆ median HMRI) had a significant improvement of LVEF at four months compared to baseline (42.7±6.9% vs. 37.5±7.9%, p=0.005). In the smaller infarct size group the effects were not observed.
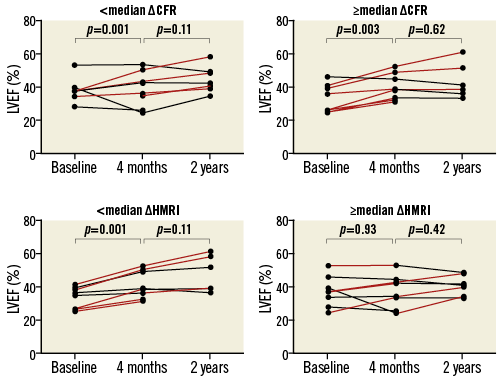
Figure 2. Change in coronary microvascular function and left ventricular function in patients with a large infarct size. Patients with a large baseline infarct size (mean infarct size 33.1±5.5% LV) were subdivided based on the mean CFR and HMRI improvement between baseline and four months. LVEF (%) between baseline and four months was compared. CFR: coronary flow reserve; HMRI: hyperaemic microvascular resistance index
Discussion
In our study, we found supporting evidence on the importance of the microvasculature status following STEMI. Microvascular dysfunction in the subacute phase, as measured with CFR, contrary to HMRI, was associated with LVEF at both four months and two years assessed with CMR. Second, an impaired CFR in the subacute phase was associated with alterations in diastolic volume. Finally, improvement in CFR was concurrently associated with improvement in LVEF between baseline and four months in patients with a large infarct size.
The added value of the current study compared to previous studies stems from the systematically obtained intracoronary Doppler flow measurements and CMR imaging at similar time points and at two years. This provides insights into the recovery of microvascular dysfunction and whether this is associated with LVF recovery. Results of previous studies support the prognostic importance of microvascular function following STEMI. These studies used different diagnostic modalities for the assessment of microvascular function, including myocardial contrast or transthoracic Doppler echocardiography5,13-15.
Bax et al reported that CFR measured directly after PPCI in anterior STEMI was the only predictor of LV improvement at six months as assessed by echocardiography. However, the mean peak CK-MB was not included in the multivariate model since the aim of the study was to assess early determinants (at the time of reperfusion) of LVF recovery. In the current study, however, CFR in the subacute phase was not independently associated with LVEF at two years but peak CK-MB was independently associated. This observation could be the result of several factors. First, the small number of patients limits the ability to perform a reliable multivariate regression model. Second, CFR measured in the subacute phase is associated with infarct size, as measured by maximum CK-MB16. From a pathophysiological perspective, the ability of the coronary microvasculature to vasodilate is related to the severity of the initial myocardial injury. In our study, we also observed that patients with large infarct sizes had a significantly lower CFR. Consequently, it is difficult to determine whether CFR is a marker of larger infarcts or whether impaired microvascular function has a primary role in adverse LV remodelling. Nonetheless, CFR in the subacute phase of STEMI is associated with LVF at both four months and two years. In order to understand these findings and implications, it is important to understand the differences between both indices. As shown in Table 2, baseline average peak velocity (APV) is probably increased due to compensatory vasodilatation, and the hyperaemic APV decreased probably secondary to microembolisation. Since myocardial infarction affects both the baseline and hyperaemic coronary flow, both these effects are taken into account by the CFR, whereas the HMRI does not consider a baseline coronary flow. This observation is in accordance with previous studies. This increase in baseline peak flow velocity has been described as being a result of coronary autoregulation17,18. This is of importance as it facilitates the compensatory vasodilatation of the coronary resistance vessels in order to maintain stable resting coronary blood flow in the distal myocardium19.
We also assessed the temporal evolution of microvascular dysfunction, as measured by the delta CFR and HMRI between baseline and four months. In the large infarct size group, this observed improvement of CFR and decrease in HMRI were both associated with recovery of LVEF. In the smaller infarct size group this association was not observed. Our findings are concordant with the studies by Suryapranata et al15 and Sezer et al20, and extend on their findings. In the first study, an increase in flow reserve documented before hospital discharge was associated with a significant improvement in global and regional LVF15. In the Suryapranata et al study, improvement in CFR in the total population was associated with a decreased infarct size on single-photon emission computed tomography and improved LVEF on echocardiography.
Dysfunction of the coronary microvasculature has also been associated with the occurrence of clinical endpoints, including heart failure and cardiac mortality21-23. Furber at al assessed whether an impaired microvascular perfusion in the IRA, as measured by a short diastolic deceleration time, is predictive of cardiac events within four years21. In addition to age and time to PPCI ≥6 hours, impaired microvascular perfusion was found to be an independent predictor, particularly for the occurrence of heart failure. Also, an impaired CFR in the reference vessel in STEMI patients has been independently associated with an increased cardiac mortality at 10 years23. These clinical implications of microvascular dysfunction following STEMI have fuelled studies on therapeutic strategies aimed at protecting the microvasculature24.
Limitations
Several limitations should be mentioned. First, intracoronary measurements were performed within one week following STEMI and the CFR may have partly recovered. Subsequently, the association between CFR in the subacute phase and LVF may have been underestimated. Second, a CFR value of 2.0 was arbitrarily chosen because of the median values of CFR in the present study.
Conclusions
Microvascular dysfunction in the subacute phase, as measured by CFR, is associated with LVF at two years in STEMI. Improvement of CFR and a decrease in HMRI within the initial months following STEMI is associated with early LVEF improvement in patients with a large infarct size, underlining the functional significance of the microvasculature after STEMI.
| Impact on daily practice The results of the current study support the growing evidence on the role of the microvasculature status following STEMI and its effect on LVF. We demonstrated that microvascular dysfunction, as measured by CFR, is associated with LV improvement in the long term. This underlines the importance of possible therapeutic strategies aimed at restoring or protecting the microvasculature following STEMI. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

