
Although early primary percutaneous coronary interventions (PCI) have dramatically improved survival in patients with ST-segment elevation myocardial infarction (STEMI), patients who develop cardiogenic shock (CS) after acute MI are often not managed with guideline-recommended therapies and mortality rates remain high. In addition, among patients who receive PCI for CS, the mortality rates appear to be increasing over time, perhaps due to treatment of higher acuity patients1. These observations underscore the need for a better understanding of the pathology and more effective treatments.
We know that PCI success is an important determinant of survival. Primary PCI in early presenting, non-shock STEMI patients achieves Thrombolysis In Myocardial Infarction (TIMI) 3 flow in more than 90% of cases. Conversely, in patients with acute myocardial infarction (AMI) complicated by CS, PCI success rates are much lower, and the lack of restoration of flow in both shock and non-shock groups is associated with worse survival1,2. However, studies have suggested that myocardial perfusion, as determined by blush scores, is a stronger predictor of survival after STEMI.
In this issue of EuroIntervention, Overtchouk et al report core laboratory-assessed TIMI flow and myocardial perfusion grade (TMPG) in 665 patients with CS enrolled in the CULPRIT-SHOCK trial3.
As expected, both TIMI flow grade and TMPG after PCI correlated with 30-day mortality, but in a multivariate model only myocardial perfusion was associated with mortality (adjusted odds ratio 0.38 [0.20-0.71], p=0.002). The authors are to be congratulated on completing this provocative trial looking at some of the angiographic criteria that affect mortality in CS patients. However, the article is entitled “Angiographic predictors ......”, yet only TIMI flow and TMPG are discussed. For interventional cardiologists, it would be useful to know if there were other angiographic predictors of poor outcome, such as significant disease proximal or distal to the treated lesion or residual thrombus. In addition, it is surprising that pre-PCI TIMI flow was not important given that it predicts smaller infarct size and survival in other studies4 and it is believed that early vessel patency reduces the risk of developing CS. It should be pointed out that 33% of the patients in this trial had TIMI 3 flow at baseline (much higher than expected from prior STEMI trials), probably because only 63% of patients in this report had STEMI. How the culprit vessel was determined in NSTEMI patients is not defined and one may expect NSTEMI patients to have greater coronary flow at baseline. Moreover, there are no data provided regarding the non-culprit vessel. Surprisingly, the multivariate analysis did not appear to consider data proven to be of prognostic importance in the investigators’ prior trial5.
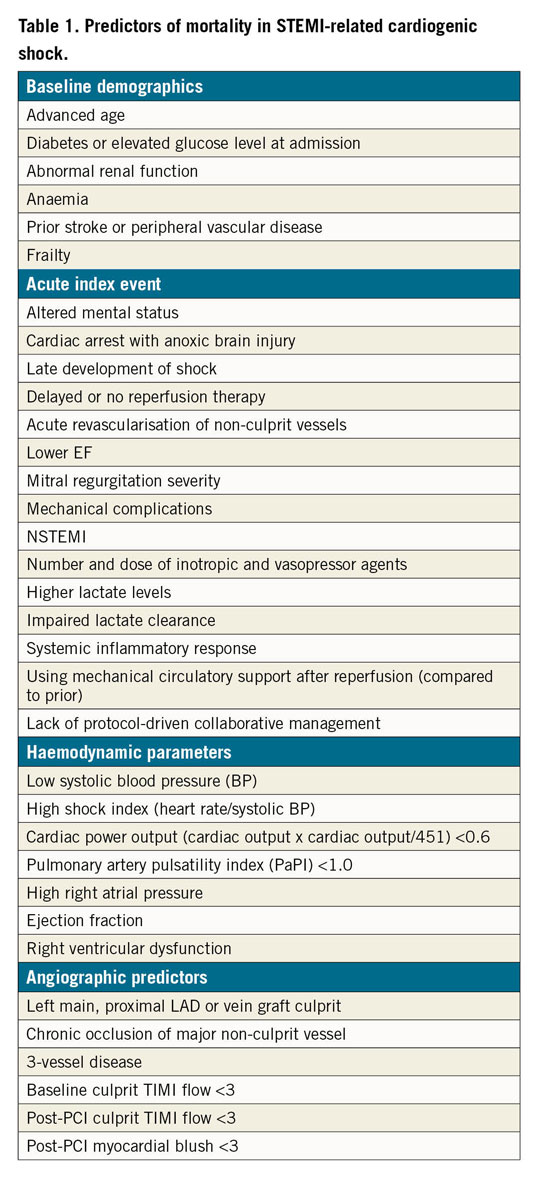
Table 1 lists risk factors that have been identified as being associated with short- and/or long-term mortality in CS1,5,6,7,8,9,10. Importantly, early identification and treatment of patients at risk but not hypotensive9 may prevent development of end-stage CS; however, we need randomised trials in Society for Cardiovascular Angiography and Interventions (SCAI) Shock Classification categories A and B in order to be certain10.
Finally, we agree that myocardial perfusion is an important prognostic indicator; however, what can be done to improve perfusion? Numerous pharmacologic agents have failed to improve infarct size or prognosis. In a recent study, the TIMI 3 flow grade following PCI was surprisingly good (91%) despite patients being in CS11. It is intriguing to think that this may be due, in part, to the improvement in cardiac output and reduction of left ventricular end-diastolic pressure (LVEDP) with mechanical circulatory support placed prior to PCI, thus improving the coronary perfusion pressure gradient. Ongoing studies, such as the Door to Unload (DTU)-STEMI trial, will hopefully provide more answers.
Conflict of interest statement
C. Grines is on the Abiomed advisory board. A. Dupont has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

