Abstract
Background: Pre-hospital platelet inhibition in patients with ST-segment elevation myocardial infarction (STEMI) may improve outcomes. RUC-4 is a novel, second-generation glycoprotein IIb/IIIa inhibitor designed for first-point-of-medical-contact treatment for STEMI by subcutaneous injection.
Aims: The open-label, phase 2A, CEL-02 trial aimed to assess the pharmacodynamics (PD), pharmacokinetics (PK), and tolerability of RUC-4 in STEMI patients undergoing primary PCI (pPCI).
Methods: A total of 27 STEMI patients received a weight-adjusted subcutaneous injection of RUC-4 before pPCI in escalating doses (0.075 mg/kg [n=8], 0.090 mg/kg [n=9], or 0.110 mg/kg [n=10]).
Results: The primary PD endpoint of high-grade (≥77%) inhibition of the VerifyNow iso-TRAP assay at 15 minutes was met in 3/8, 7/8, and 7/8 patients in the three cohorts with a dose-response relationship (mean inhibition [min - max] of 77.5% [65.7%-90.6%], 87.5% [73.8%-93.1%], and 91.7% [76.4%-99.3%], respectively; ptrend=0.002). Fifty percent (50%) inhibition remained after 89.1 (38.0-129.7), 104.2 (17.6-190.8), and 112.4 (19.7-205.0) minutes. Injection site reactions or bruising were observed in 1 (4%) and 11 (41%) patients, respectively. Mild access-site haematomas occurred in 6 (22%), and severe access-site haematomas occurred in 2 patients (7%). No thrombocytopaenia was observed within 72 hours post dose.
Conclusions: In patients with STEMI, a single subcutaneous dose of RUC-4 at 0.075, 0.090, and 0.110 mg/kg showed dose-response high-grade inhibition of platelet function within 15 minutes.
Introduction
Platelet activation and thrombus formation play a pivotal role in the pathophysiology of acute coronary syndromes (ACS). Current guidelines recommend treatment of ACS patients with dual antiplatelet therapy (DAPT, aspirin and a P2Y12 inhibitor) to reduce thrombotic events1. However, the onset of action of oral P2Y12 inhibitors is slow, often requiring as long as 4-6 hours in the setting of ST-segment elevation myocardial infarction (STEMI)2, independent of higher loading doses3. Late onset of action of oral P2Y12 inhibition may be due to delayed gastrointestinal absorption, cardiogenic shock, vomiting of the loading dose, or concomitant use of opioids4,5. Despite the faster platelet inhibition by either chewed or crushed oral P2Y12 inhibitors, an initial gap in the optimal platelet inhibition still exists, especially in the crucial first hours of STEMI6,7,8,9.
Early, parenteral platelet inhibition with glycoprotein IIb/IIIa inhibitors (GPI) in STEMI patients has been shown to improve pre-primary percutaneous coronary intervention (pPCI) target vessel patency, post-pPCI ST-segment resolution, and patient outcomes10,11,12,13. However, conventional GPI are associated with increased bleeding and thrombocytopaenia14,15. They also require continuous intravenous (IV) infusion, which is inconvenient in the acute, pre-hospital phase. Hence, there is an unmet need in the acute phase of STEMI for easily administered platelet inhibitors that on the one hand are rapidly acting and potent, thereby reaching maximal efficacy quickly, and on the other hand have limited duration of action, thereby reducing the risk of bleeding.
RUC-4 is a novel, small-molecule, second-generation GPI that was designed for first-point-of-medical-contact treatment for STEMI – administered subcutaneously by a single injection16,17 and potentially by auto-injector. In Phase 1 studies in healthy subjects and patients with stable coronary artery disease (CAD) treated with aspirin, RUC-4 was well tolerated up to 0.075 mg/kg and achieved high-grade inhibition of platelet function measured by light transmission aggregometry (LTA) in response to 20 µM adenosine diphosphate (ADP) within 15 minutes, with return of platelet function within two hours18,19. Previous studies of GPI found that 80% inhibition of LTA stimulated with 20 µM ADP correlated with in vivo antithrombotic effects in patients undergoing PCI and improved outcomes20.
As the next step in the development of RUC-4, we conducted an open-label, dose escalating, Phase 2A study (CEL-02) to assess the pharmacodynamic (PD) and pharmacokinetic (PK) properties of a weight-adjusted dose of RUC-4 that was administered to STEMI patients in the cardiac catheterisation laboratory (CCL). Furthermore, the tolerability of RUC-4 will be described in this population.
Methods
This was a prospective, open-label, single-centre, Phase 2A study of a single subcutaneous dose of RUC-4 in patients with STEMI undergoing pPCI. The study was conducted at the St. Antonius Hospital, Nieuwegein, the Netherlands, approved by the local independent ethics committee, and conducted in compliance with the principles of the Declaration of Helsinki, Good Clinical Practice, and local laws and regulations. The study is registered on ClinicalTrials.gov (NCT04284995). The full study protocol is attached in Supplementary Appendix 1. All patients provided verbal consent on arrival at the CCL prior to any study procedures; written informed consent was obtained before hospital discharge.
Based on the PK/PD data from the Phase 1 study, patients were enrolled in three cohorts of eight patients, and received a weight-adjusted dose of RUC-4, starting at 0.075 mg/kg, with dose escalation to 0.090 mg/kg and 0.110 mg/kg in the second and third cohort, respectively18. Dose escalation was based on a review by the Safety Review Committee after assessing the results from eight evaluable patients, each with at least 15 days of follow-up.
STUDY PATIENTS
Eligible patients were adults presenting for pPCI with STEMI within six hours of symptom onset. Patients were not eligible if they met one of the following criteria: high suspicion of a stent thrombosis causing the current STEMI; high suspicion of type 2 myocardial infarction (MI); out-of-hospital cardiac arrest; cardiogenic shock; persistent severe hypertension; severe renal dysfunction; clinically important anaemia; coagulation abnormality; bleeding disorder; prior haemorrhagic or ischaemic stroke; estimated body weight <52 kg or >120 kg; or use of concomitant IV GPI or oral anticoagulants. The complete inclusion and exclusion criteria are listed in the study protocol (Supplementary Appendix 1).
STUDY PROCEDURES
All patients received standard of care treatment for STEMI in the ambulance, including aspirin (500 mg IV), ticagrelor (180 mg oral), and unfractionated heparin (UFH; 5,000 IU IV). Upon arrival at the CCL, after consent, a pre-dose blood sample was obtained, and patients were administered a single subcutaneous dose of RUC-4 in the outer aspect of the upper arm. If UFH was administered pre-hospital, an activated clotting time (ACT) was measured and, if the ACT was <200 seconds, additional UFH was recommended. For PD/PK assessments, additional blood samples were obtained at 15, 30, 45, 60, 90, 120 and 180 minutes post dose; a 240-minute PD time point was added for the second and third cohorts. Platelet counts were assessed up to 72 hours post dose.
PHARMACODYNAMIC ASSESSMENTS
Samples were collected in vacuum tubes (Greiner Bio-One 2 mL, 3.2% sodium citrate) for platelet function measurements. The primary PD endpoint of inhibition of platelet function was measured with the VerifyNow P2Y12 assay employing the thrombin receptor activating peptide (iso-TRAP) channel21 with the objective of achieving 77% or greater inhibition of platelet function at 15 minutes. In studies submitted for publication (Bentur et al), we found that 77% inhibition of the iso-TRAP channel corresponds to 80% inhibition of LTA stimulated by 20 µM ADP, the value that has been most closely correlated with antithrombotic effects in vivo using other GPI20 (Supplementary Appendix 2). We also determined that the iso-TRAP assay was not affected by aspirin, and only inhibited by about 20% at peak levels of ticagrelor achieved in vivo. We also assessed the results from another channel in the P2Y12 cartridge, termed the Base channel, which contains a higher concentration of iso-TRAP than the iso-TRAP channel (20 µM vs 3-4 µM) in addition to an activator of an additional thrombin receptor (PAR-4). We found that this assay is not affected by either aspirin or ticagrelor, even at peak levels of ticagrelor achieved in vivo, and that 77% inhibition of platelet function using this assay is also the equivalent of 80% inhibition of 20 µM ADP-induced LTA. The time to return to 50% platelet function was reported for the iso-TRAP and Base assays as a measure of the offset of RUC-4’s antiplatelet effects.
PHARMACOKINETIC ASSESSMENTS
Samples for PK analysis were collected by adding 1 mL of whole blood to tubes containing 4 mL of pre-chilled 30% acetonitrile. After vortexing, samples were frozen at –80°C within two hours of blood collection. Samples from each cohort were analysed in batches as previously described18. PK parameters for RUC-4 and its most abundant metabolite, RUC-4-des-glycine, were calculated. For the non-compartmental PK analysis, all blood concentration levels below the level of quantification occurring prior to the first measurable concentration were set to zero. Values below the level of quantification occurring after the first measurable blood concentration were noted as missing. PK data reported include the whole blood RUC-4 concentration (ng/mL), the maximal RUC-4 concentration (Cmax), and time to reach maximum RUC-4 concentration (tmax).
TOLERABILITY
Platelet counts were measured pre-dose and at 1, 24, and 72 hours post dose. Bleeding events were classified according to the Bleeding Academic Research Consortium (BARC) criteria22; intraprocedural thrombosis and injection site reactions were assessed up to 30 days post dose. All adverse events were classified Grade 1 to 5 as per Common Terminology Criteria for Adverse Events version 5.0.
STATISTICAL ANALYSIS
All statistical summaries are descriptive in nature. No hypothesis testing was performed for primary and secondary analyses.
For continuous data, summary statistics including number of observations (n), arithmetic mean and standard deviation (SD), median, quartile 1 (Q1), quartile 3 (Q3), minimum (min), and maximum (max) are presented. For categorical data, frequency counts and percentages are presented.
Descriptive statistics are presented for the PD/PK data at different time points and depicted graphically. A ptrend was calculated for percent inhibition at 15 minutes. An inverse estimation was performed for return to 50% platelet inhibition, Cmax and tmax based on a mixed model with a continuous time effect and random intercept. Statistical analyses were performed using SAS, release 9.4 (SAS Institute, Cary, NC, USA).
Results
BASELINE CHARACTERISTICS
Between June 2020 and October 2020, 27 patients were enrolled and received RUC-4 (0.075 mg/kg [n=8], 0.090 mg/kg [n=9] or 0.110 mg/kg [n=10]), upon arrival at the catheterisation laboratory, immediately before pPCI. Of these, 24 patients were evaluable for PD analysis and 25 were evaluable for PK analysis (Central illustration). The reasons for being unevaluable were: withdrawal of informed consent (one patient, excluded for PK and PD), failed baseline sampling (one patient, excluded for PD), and use of wrong VerifyNow cartridges (one patient, excluded for PD).
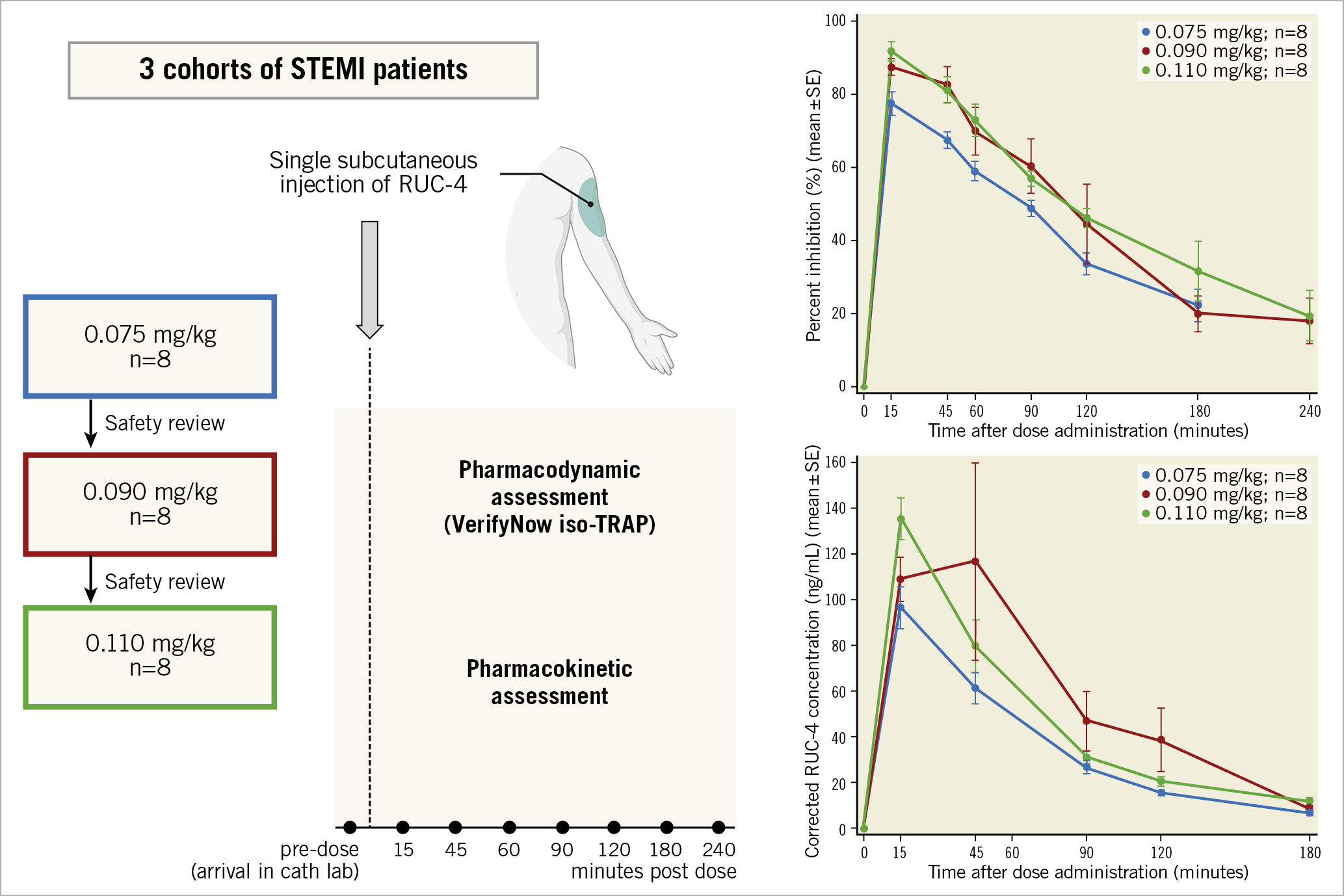
Central illustration. Single-dose subcutaneous RUC-4 induces a fast, potent, dose-dependent platelet inhibition in STEMI patients presenting for primary PCI. PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TRAP: thrombin receptor activating peptide
Patient demographics, baseline characteristics, and concomitant medications are summarised in Table 1. The majority of patients were male (74%) and Caucasian (96%); their mean age was 62 (range 38-91) years. All patients presented with symptoms and ECG findings consistent with STEMI; however, in one patient ST-segment elevation was caused by perimyocarditis without CAD, and one patient had a spontaneous coronary artery dissection with spasm resulting in <50% stenosis. Thus, 25/27 (93%) patients were treated with pPCI for an acute coronary occlusion.
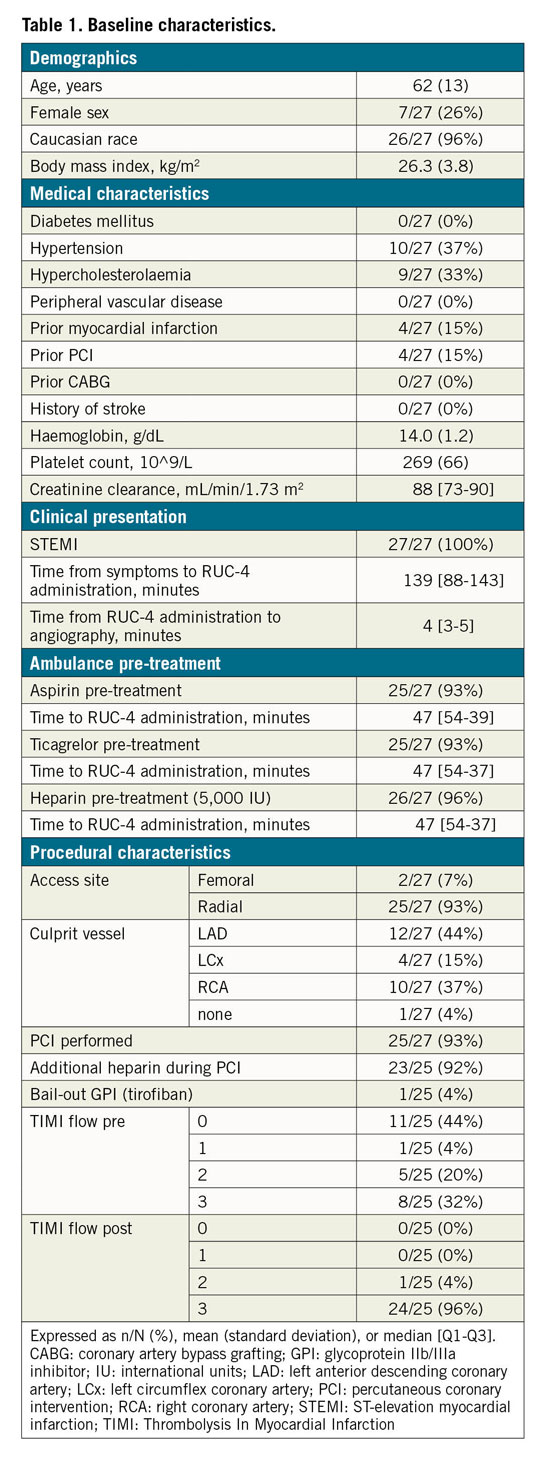
Almost all patients were concomitantly treated with aspirin (93%), ticagrelor (93%), and UFH (96%) in the ambulance. The ACT prior to pPCI was <200 seconds in 92% of the patients, and thus they received additional UFH during cardiac catheterisation. Flow was completely restored (Thrombolysis In Myocardial Infarction [TIMI] 3 flow) in 96% of patients who underwent PCI. One patient had a suboptimal result of PCI and a high thrombus load, and so received tirofiban bail-out treatment ~90 minutes later, at a time when the platelet inhibitory effect of RUC-4 was expected to be less than 50%.
PHARMACODYNAMICS
Figure 1 shows the platelet function findings 15 minutes after receiving RUC-4 for each patient in each cohort using both the iso-TRAP and Base channels. RUC-4 produced greater than 77% inhibition of platelet function in 3/8, 7/8, and 7/8 patients in the subsequent cohorts with the iso-TRAP assay, and 7/8, 8/8 and 8/8 patients reached greater than 77% inhibition of platelet function with the Base channel assay, respectively. The one patient in the third cohort who did not have 77% or more inhibition of platelet function with the iso-TRAP assay at 15 minutes had 76% inhibition of platelet function. The inhibition of platelet function was greatest at 15 minutes (Figure 2, Figure 3) and then began to decrease, reaching 50% inhibition on the iso-TRAP assay at 89.1 (95% confidence interval [CI]: 38.0-129.7), 104.2 (95% CI: 17.6-190.8) and 112.4 minutes (95% CI: 19.7-205.0) after administration in the cohorts, respectively. The comparable times with Base channel assay were 89.1 (95% CI: 36.9-141.3), 120.8 (95% CI: 6.2-235.4) and 128.9 minutes (95% CI: 41.6-216.2).
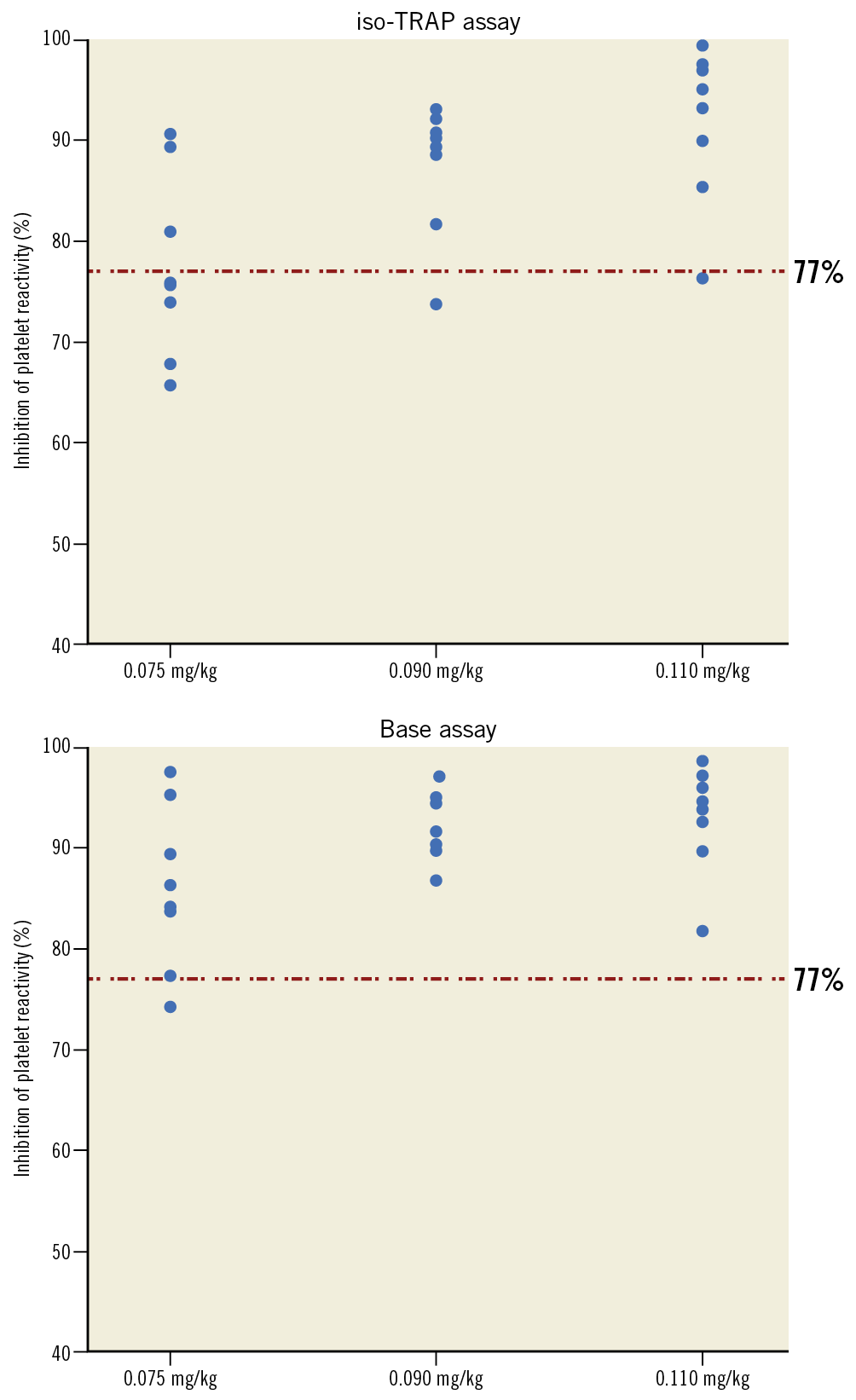
Figure 1. Pharmacodynamic response at 15 minutes post dose to different doses of RUC-4. Inhibition of platelet reactivity, assessed by VerifyNow iso-TRAP (A) and Base (B, iso-TRAP + PAR-4) assays 15 minutes following subcutaneous injection of RUC-4 at 0.075, 0.090, and 0.110 mg/kg. PAR-4: protease-activated receptor 4; TRAP: thrombin receptor activating peptide
Figure 2 and Figure 3 provide the aggregate inhibition of platelet function data with the iso-TRAP and Base channel assays, respectively, at all of the indicated time points. For the iso-TRAP assay, the mean (min-max) results at 15 minutes were 77.5% (65.7%-90.6%), 87.5% (73.8%-93.1%), and 91.7% (76.4%-99.3%), respectively (ptrend=0.002). The Base channel inhibition of platelet function at 15 minutes post dose with the three cohorts was 86.0% (74.2%-97.7%), 92.3% (86.8%-97.1%), and 93.0% (81.9%-98.6%), respectively (ptrend=0.029).
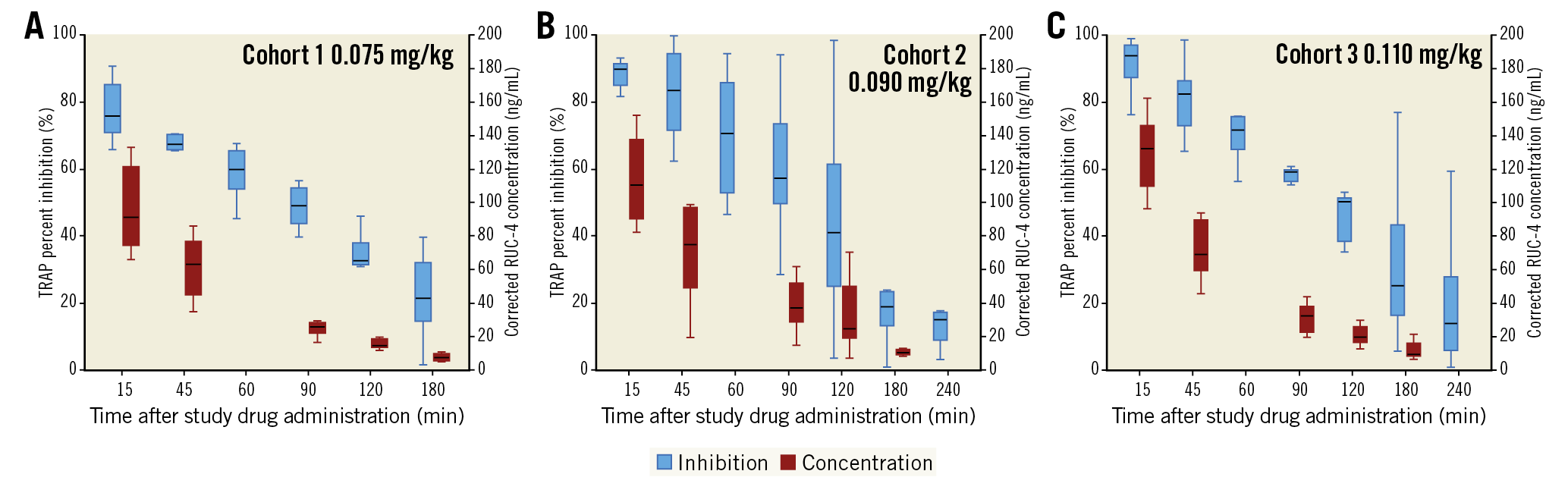
Figure 2. RUC-4 pharmacokinetics and pharmacodynamics using the iso-TRAP assay. Platelet reactivity, assessed by VerifyNow and expressed as percent inhibition of the iso-TRAP assay (blue box plots) up to 180 min (cohort 1) or 240 min (cohorts 2 and 3), and plasma concentration of RUC-4 (red) up to 180 min (cohorts 1, 2, and 3) following subcutaneous injection of RUC-4 at 0.075 mg/kg (A), 0.090 mg/kg (B) and 0.110 mg/kg (C). Box plots represent the lower (25th) quartile, median, and upper (75th) quartile. IQR: interquartile range; TRAP: thrombin receptor activating peptide
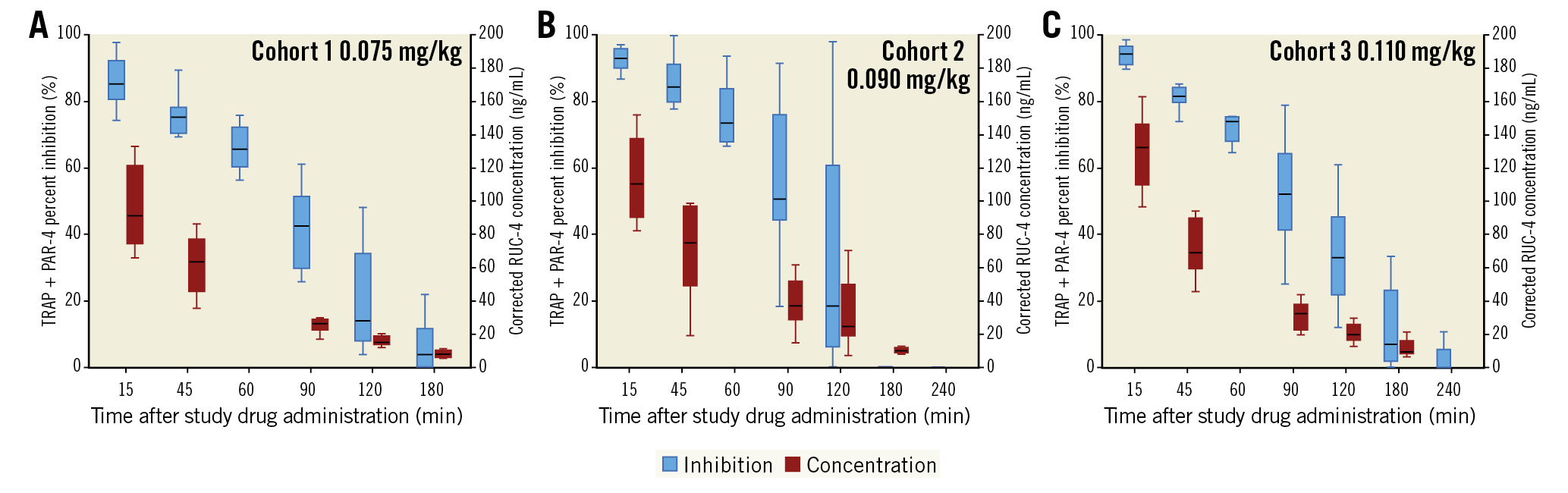
Figure 3. RUC-4 pharmacokinetics and pharmacodynamics using the Base assay. Platelet reactivity, assessed by VerifyNow and expressed as percent inhibition of the Base assay (blue box plots) up to 180 min (cohort 1) or 240 min (cohorts 2 and 3), and plasma concentration of RUC-4 (red) up to 180 min (cohorts 1, 2, and 3) following subcutaneous injection of RUC-4 at 0.075 mg/kg (A), 0.090 mg/kg (B) and 0.110 mg/kg (C). Box plots represent the lower (25th) quartile, median, and upper (75th) quartile. IQR: interquartile range; PAR-4: protease-activated receptor 4; TRAP: thrombin receptor activating peptide
PHARMACOKINETICS
Following the RUC-4 injection a mean maximum blood concentration of (mean±SD) 96.7±26.0 ng/mL, 152.4±114.0 ng/mL, and 129.8±23.3 ng/mL was observed for the 0.075 mg/kg, 0.090 mg/kg, and 0.110 mg/kg cohorts, respectively (Figure 2, Figure 3). These maximum whole blood concentrations were reached at mean 20 minutes post dose (tmax, median [min-max]: 15 [15-16] minutes, 15 [14-45] minutes, and 15 [13-45] minutes, respectively).
TOLERABILITY
None of the patients included in the study developed thrombocytopaenia during the first 72 hours after the study drug administration. In the safety cohort, which included all patients receiving RUC-4 whether their data were evaluable or not, 25/27 (93%) reported at least one adverse event (AE) (Table 2). The most common AEs were bruising at the RUC-4 injection site (41%) and vascular access-site haematoma or bruising (30%). The majority (69%) of these were rated as grade 1 (11/16) or grade 2 (3/16).
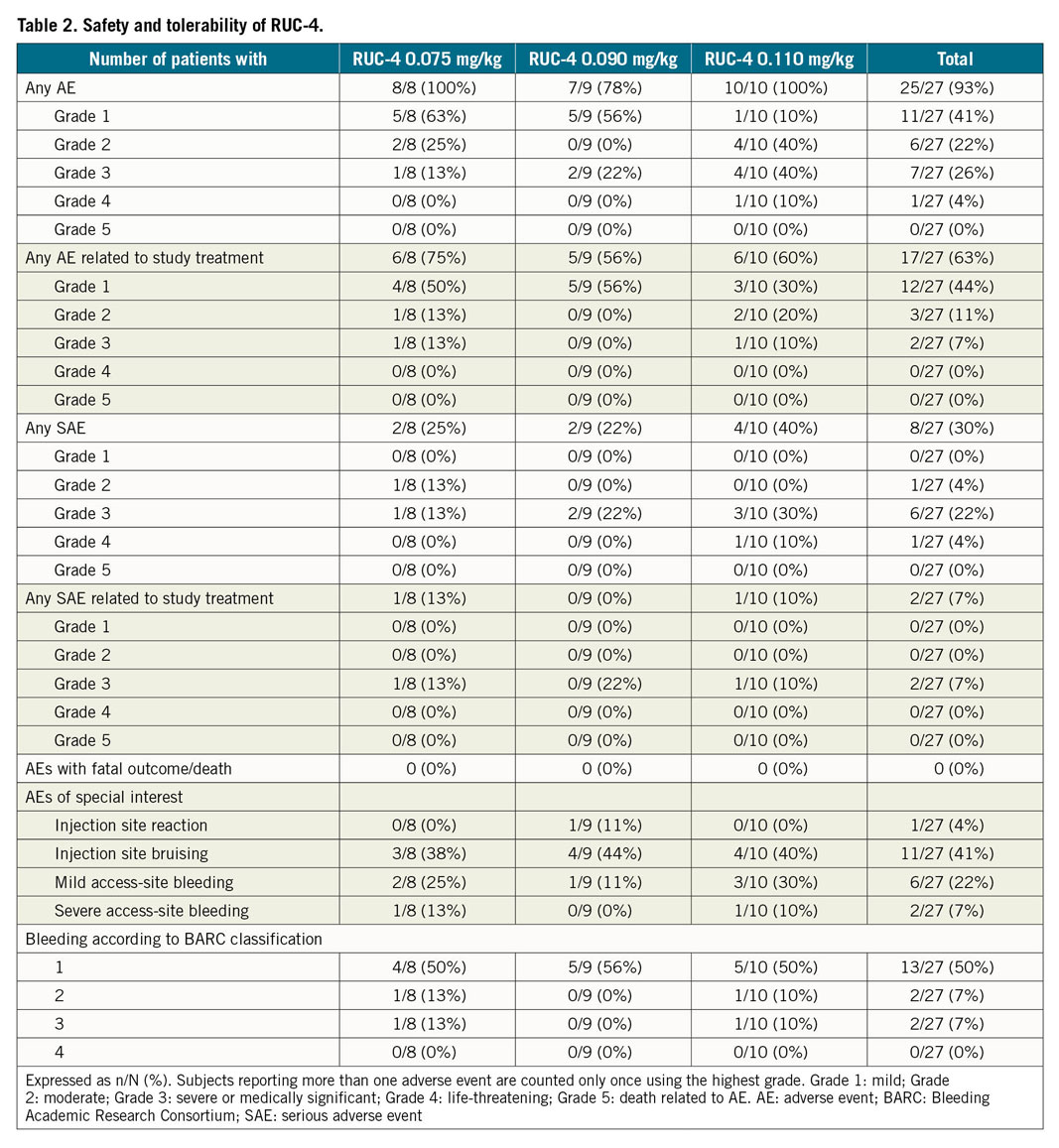
Eleven serious adverse events (SAEs) occurred in 8/27 patients (30%), of which 9/11 (82%) were judged not to be related to RUC-4. Two SAEs were assessed as probably related to RUC-4, but did not show a dose relationship, with one in a patient in the 0.075 mg/kg cohort and the other in the 0.110 mg/kg cohort. In both cases, the SAE was access-site bleeding from the radial artery (BARC type 3a and 3b). In 13 patients at least one BARC 1, and in two patients one or more BARC 2 bleeding was reported. One transient injection site reaction was reported, which was mild.
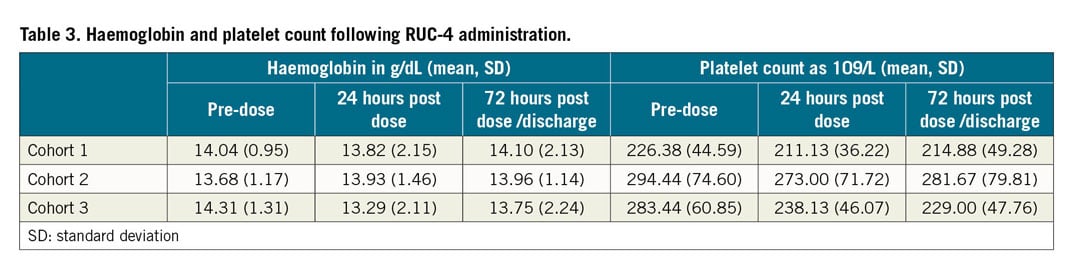
There were no clinically meaningful changes in blood counts (Table 3), or in kidney or liver function analytes. No deaths occurred during the study period.
Discussion
In the current Phase 2A study we analysed the PD and PK properties of RUC-4 in 27 patients with STEMI undergoing pPCI. The most important observations were: 1) RUC-4 rapidly achieved dose-dependent high-grade inhibition of platelet function, reaching a level that has been shown to correlate with improved clinical outcomes in patients treated with other GPI10,11,12,20; 2) the inhibition of platelet function declined after the 15-minute time point, reaching ~50% inhibition of platelet function between 90 and 120 minutes after administration in a dose-dependent manner; 3) the whole blood levels of RUC-4 correlated closely with the inhibition of platelet function and the correlation was consistent with the RUC-4 IC50 values for inhibiting platelet aggregation established in vitro16,19; 4) RUC-4 did not produce thrombocytopaenia in the first 72 hours post dose; and 5) the majority of bleeding/bruising complications were grade 1 or 2; there were two bleeding events classified as BARC 3a and 3b.
The earlier Phase 1 study of RUC-4 established its ability consistently to achieve high-grade inhibition of platelet function within 15 minutes of subcutaneous administration in healthy volunteers and in patients with stable CAD on aspirin18. The results of the current study are important since STEMI patients undergoing pPCI may differ in their response to RUC-4 from those healthy volunteers studied in Phase 1. They are concomitantly treated with aspirin, ticagrelor, and heparin. Also, pharmacokinetic responses may be altered by STEMI-induced selective shunting of blood to the vital organs, causing decreased perfusion of the skin and thus uptake of the investigational product. Despite these theoretical concerns, the PK/PD results in this study are similar to those in the Phase 1 study in healthy volunteers and patients with stable CAD on aspirin18. Patients with cardiogenic shock were excluded from our study, so it is unclear whether it will have an effect on RUC-4’s PK/PD.
There is a need for drugs such as RUC-4 in the early treatment of patients with STEMI because the effect of oral P2Y12 inhibition is delayed, especially in these STEMI patients2. Despite the faster platelet inhibition by either chewing or crushing oral P2Y12 inhibitors6,7,8, a gap of several hours in optimal platelet inhibition remains; until now, no study investigating oral P2Y12 inhibition has shown a clinical benefit in STEMI patients8.
In contrast, the use of GPI has been validated as an effective therapy for MI patients undergoing pPCI23, and in meta-analyses of early administration in the ambulance or emergency department has been shown to be superior to in-hospital administration in reperfusing the target artery, improving ST-segment resolution, left ventricular function, and mortality12,13. Despite these data, GPI are not routinely administered in the ambulance or emergency department, in part because they require IV administration as a bolus and continuous infusion controlled by a pump. Also, the reduction of thrombotic events was counterbalanced by more severe bleeding in patients treated with GPI23, although some of this bleeding has been ameliorated by the increased use of radial artery access24.
In this study, two severe access-site bleedings were judged by the PCI operators to be most likely due to catheter-based trauma to the proximal radial artery. As it is a potent antiplatelet agent, the investigators judged the haematomas probably to be related to RUC-4 treatment; however, it was difficult to assess its precise contribution since, in addition to the presumed arterial injury, the patients were treated with aspirin, heparin, and ticagrelor, all of which are also known to increase bleeding. Overall, the study was not designed or powered to evaluate safety.
In addition, all of the current GPI are associated with thrombocytopaenia in a small percentage of patients (0.5%-2%)15. Since RUC-4 is unique among small molecule GPI in not inducing the receptor to undergo a major conformational change that has been implicated in the development of thrombocytopaenia25, it is possible that RUC-4 may be associated with fewer episodes of thrombocytopaenia than current GPI. Additional studies will be required to assess this hypothesis.
The only approved parenteral P2Y12 inhibitor, cangrelor, is effective in achieving rapid platelet inhibition26 but, like currently available GPI, it requires continuous IV administration, whereas RUC-4 is administered by a single subcutaneous dose. Moreover, even the most potent P2Y12 inhibitors are less effective in inhibiting platelet function than GPI because they only inhibit one activation pathway (ADP-initiated), whereas GPI block the final pathway downstream of all of the activation pathways9,27. Furthermore, cangrelor is not currently recommended for pre-hospital use; rather it is used in patients who do not receive a P2Y12 inhibitor before the procedure or in those considered unable to absorb oral agents28.
The antiplatelet effects of RUC-4 wear off rapidly, reaching about 50% inhibition between 90 and 120 minutes after administration. This reduces the time during which there is high-grade inhibition of platelet function and thus should reduce the risk of haemorrhage relative to the current agents that inhibit the receptor for longer periods of time. The primary goal with RUC-4 is to initiate immediate reperfusion as rapidly as possible and prevent re-occlusion before and during the pPCI. In the current study, the time from administration of aspirin, ticagrelor, and heparin to pPCI was 50±16 minutes, a time period during which RUC-4 produced greater than 50% inhibition of platelet function. Thus, the optimal time for the effects of RUC-4 to dissipate will depend on the local metrics with regard to performing the PCI. Our data show that the higher doses of RUC-4 not only achieve greater inhibition of platelet function at 15 minutes, but also achieve longer duration of inhibition of platelet function. Thus, if it is deemed more desirable to prolong the effect of RUC-4, this can be accomplished by increasing the dose. This will be studied in the upcoming Phase 2B CELEBRATE trial.
Another experimental subcutaneously administered P2Y12 inhibitor, selatogrel, has been investigated in patients with MI29. Forty-seven patients were randomised to a single subcutaneous dose prior to PCI. Selatogrel inhibited the VerifyNow P2Y12 assay, but no data were provided on its ability to inhibit TRAP-induced platelet aggregation. Selatogrel’s effects last for at least eight hours, which will also affect its efficacy and safety profile. Data from other P2Y12 antagonists, including the potent prasugrel, indicate that doses that cause near-complete inhibition of the P2Y12 assay do not inhibit the Base channel assay30. In sharp contrast, within 15 minutes, RUC-4 inhibited the Base channel assay on average by more than 90%. Clinical studies are required to assess whether the enhanced in vitro antiplatelet effects of RUC-4 will translate into improved clinical outcomes. It is also possible that combining RUC-4 and selatogrel may be beneficial.
Limitations
Our study has several limitations. First, it had an open-label design without a placebo, but this design is unlikely to influence the PD and PK data of our study. Second, as currently configured, the iso-TRAP and Base channel assay data are not reported by the VerifyNow instrument and so they needed to be calculated from the raw data obtained from the instrument. Thus, they are currently not commercially available. VerifyNow assays have the advantage of being automated and so are much easier to perform in the emergency setting than the labour-intensive and difficult-to-standardise LTA, the current gold standard of platelet function testing. Since in vivo antithrombotic effects and clinical outcomes have correlated closely with ADP-induced LTA20, we calibrated these assays against ADP-induced LTA (Supplementary Appendix 2) and found that RUC-4 achieves dose-dependent inhibition of platelet function comparable to the LTA standard of 80% within 15 minutes after subcutaneous administration.
The timing of subcutaneous injection and start of the PCI does not fully resemble the expected use of RUC-4 in clinical practice. To maintain control over the administration of RUC-4 until more is known about its effects in STEMI, in the current design, the aspirin, ticagrelor, and heparin were administered about 50 minutes before RUC-4 and the PCI was initiated immediately after the administration of RUC-4. The future intended application of RUC-4 would have it administered at the same time as the aspirin, ticagrelor, and heparin, and about an hour before the PCI. Thus, the peak of RUC-4’s antiplatelet effect in this study occurred at a time point when ticagrelor’s effect was already beginning to become manifest, potentially contributing to the risk of bleeding. In addition, RUC-4 had little time in which to achieve its antithrombotic effects prior to PCI and a greater chance of causing bleeding during PCI. This may be responsible for some of the bleeding events. When used according to the future intended application, the antiplatelet effects of RUC-4 will be less prominent at the time of vascular closure, and thus will probably contribute less to access-site bleeding complications.
Conclusions
In this phase 2A study in patients with STEMI, a single subcutaneous dose of RUC-4 induced dose-dependent maximal platelet function inhibition within 15 minutes, with 50% return of platelet function roughly over 90 minutes, increasing with higher doses. The results of this study warrant further research on pre-hospital treatment with RUC-4 as a bridge to onset of oral antiplatelet agents.
|
Impact on daily practice RUC-4 provides fast and potent platelet inhibition by a single subcutaneous injection. Thus, it may offer advantages compared to intravenous GPI and (oral) P2Y12 inhibitors for first-point-of-care treatment of STEMI. Its short duration of effect is designed to initiate target vessel reperfusion medically en route to PCI, while limiting the risk of bleeding during and after the procedure. The results of this study warrant further research on pre-hospital treatment with RUC-4. An upcoming Phase 2B trial will assess the efficacy and safety of RUC-4 administration to STEMI patients in the ambulance. |
Acknowledgements
The authors thank all patients, study investigators, study staff, CCL staff and nursing teams for their participation in this study. The authors acknowledge E. Kolkman, an employee of Diagram B.V., for providing statistical support during manuscript development. The authors also acknowledge the contribution of F.W.A. Verheugt for his independent role in the Safety Review Committee.
Funding
This study was supported by a grant and materials provided by CeleCor Therapeutics.
Conflict of interest statement
M. Gibson receives research support from Johnson & Johnson. He receives consulting support from AstraZeneca, Johnson & Johnson, and Janssen & Bayer. C. Granger reports consultancy or research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CeleCor, Daiichi Sankyo, Janssen, Pfizer, Novartis, NHLBI, and the FDA. O. Bentur reports research fees from CeleCor and Pulmoquine Therapeutics. B.S. Coller receives royalties from the sales of abciximab (Centocor/Janssen) and the VerifyNow assays (Accumetrics/Instrumentation Laboratories). He is also an inventor of RUC-4, a founder and equity holder in CeleCor, and a consultant to CeleCor. B.S. Coller served as a non-voting scientific consultant to the Safety Review Committee and was supported in part by grant 19278 from the National Heart, Lung and Blood Institute and Clinical and Translational Science grant UL1 TR001866 from the National Center for Advancing Translational Science. A.W.J. van ‘t Hof reports institutional unrestricted grants from Medtronic, Boehringer Ingelheim, AstraZeneca and Abbott, not related to this work. J.M. ten Berg reports lecture or consultancy fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, The Medicines Company, AccuMetrics, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Bayer, Ferrer, and Idorsia. He received institutional research grants from ZonMw, and AstraZeneca. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

