Abstract
BACKGROUND: The effect of administering a crushed prasugrel loading dose is uncertain in patients presenting with a large myocardial infarction and ST-segment elevation myocardial infarction (STEMI).
AIMS: The aim of this study was to investigate if patients with a large myocardial infarction may benefit from prehospital administration of a crushed prasugrel loading dose.
METHODS: Patients from the CompareCrush trial with an available ambulance electrocardiography (ECG) were included in the study. An independent core laboratory confirmed a prehospital large myocardial area. We compared pre- and postprocedural angiographic markers, including Thrombolysis in Myocardial Infarction (TIMI) 3 flow in the infarct-related artery, high thrombus burden, and myocardial blush grade 3, in STEMI patients with and without a prehospital large myocardial area.
RESULTS: Ambulance ECG was available for 532 patients, of whom 331 patients were identified with a prehospital large myocardial area at risk. Crushed prasugrel significantly improved postprocedural TIMI 3 flow rates in STEMI patients with a prehospital large myocardial area at risk (92% vs 79%, odds ratio [OR] 3.00, 95% confidence interval [CI]: 1.50-6.00) but not in STEMI patients without a prehospital large myocardial area at risk (91% vs 95%, OR 0.47, 95% CI: 0.14-1.57; pinteraction=0.009).
CONCLUSIONS: Administration of crushed prasugrel may improve postprocedural TIMI 3 flow in STEMI patients with signs of a large myocardial area at risk on the ambulance ECG. The practice of crushing tablets of prasugrel loading dose might, therefore, represent a safe, fast and cost-effective strategy to improve myocardial reperfusion in this high-risk STEMI subgroup undergoing primary percutaneous coronary intervention.
Timely mechanical reperfusion therapy in patients presenting with ST-segment elevation myocardial infarction (STEMI) effectively reduces myocardial infarct size and mortality and, therefore, represents the current standard of treatment1. Platelet activation and aggregation play a pivotal role in the extent of myocardial infarct size in STEMI patients234. The extent of the infarct size, assessed with electrocardiography (ECG) or cardiac magnetic resonance imaging (CMR), has been established as a strong predictor for poor postprocedural reperfusion success and mortality567. As a result, substantial efforts have been made to reduce infarct size, including the setup of national, 24/7, prompt, responsive STEMI networks; the setup of nationwide percutaneous coronary intervention (PCI) facilities to enable timely mechanical revascularisation; and the introduction of prehospital pharmacological treatment regimens5.
The randomised COMPARison of Pre-hospital CRUSHed vs. Uncrushed Prasugrel Tablets in Patients With STEMI Undergoing Primary Percutaneous Coronary Interventions (CompareCrush) trial investigated whether early and potent platelet inhibition – facilitated by the administration of crushed prasugrel loading dose tablets in the ambulance – could improve early epicardial and myocardial perfusion in STEMI patients scheduled for primary PCI89. While administration of a crushed prasugrel loading dose significantly reduced early platelet reactivity during primary PCI, it failed to meet the trial’s primary endpoints of improving markers of early myocardial reperfusion10. Whether early, enhanced platelet inhibition could benefit the subgroup of high-risk patients who present with a large myocardial infarction area at risk on the ambulance ECG is currently unclear. Therefore, we investigated the efficacy of administering crushed versus integral tablets of prasugrel loading dose – and subsequent increased early platelet inhibition – in patients presenting with a prehospital large myocardial area at risk, reflected by the cumulative ST-segment deviation on the ambulance ECG.
Methods
STUDY DESIGN AND POPULATION
The CompareCrush trial (ClinicalTrials.gov: NCT03296540) was an investigator-initiated, multicentre, randomised ambulance trial performed in the region of Rotterdam, the Netherlands. The trial was conducted according to the Declaration of Helsinki, the Medicinal Research Involving Human Subjects Act (WMO), and the Good Clinical Practice guidelines. The study protocol and all study procedures were approved by the local ethics committee.
The trial design, as well as the inclusion and exclusion criteria, have been previously reported89. In brief, patients presenting with symptoms suggestive of myocardial infarction and new persistent ST-segment elevation of ≥1 mm in two or more contiguous leads on the ambulance ECG were eligible for study enrolment. Patients were randomly assigned to receive a 60 mg loading dose of either crushed or integral tablets of prasugrel in the ambulance. In addition, all patients received – as per the national ambulance protocol – 500 mg of aspirin and 5,000 units of unfractionated heparin, both administered intravenously. After pharmacological management, patients were transferred to an intervention centre to undergo emergency coronary angiography. The use of manual thrombus-aspiration devices and glycoprotein IIb/IIIa inhibitor bailout therapy were left to the discretion of the operator. All patients received routine postprocedural medical care. Deferred informed consent was obtained in all patients within four hours after enrolment.
The primary endpoints of the CompareCrush trial were two surrogate markers of early epicardial and myocardial reperfusion: 1) Thrombolysis in Myocardial Infarction (TIMI) 3 flow in the infarct-related artery (IRA) at initial angiography and 2) complete ST-segment resolution (STR) 1 hour post-PCI. Secondary outcomes included pharmacodynamic measurements, other angiographic parameters (e.g., TIMI thrombus grade, TIMI myocardial blush grade, etc.), and clinical outcomes at 30 days and 1 year. The main results of the CompareCrush trial showed that crushed compared with integral prasugrel administration was unsuccessful in facilitating early myocardial reperfusion, despite a significant increase in early platelet inhibition in the crushed prasugrel group. Furthermore, no differences regarding other secondary outcomes were found between groups11.
For the present substudy, we included all patients who were enrolled in the CompareCrush trial if their diagnostic ambulance ECG was retrieved and available for analysis. Patients were excluded if their ambulance ECG was not retrieved (n=59), or when the ambulance ECG showed intraventricular conduction delay (i.e., left or right bundle branch blocks or non-specific interventricular conduction delay) and therefore could not be analysed (n=42). The final substudy cohort included 532 patients.
ELECTROCARDIOGRAPHY ANALYSIS
In each patient, a 12-lead ambulance ECG was performed upon first medical contact with the ambulance service. All ECGs were reviewed by an independent, blinded ECG core laboratory. ST-segment deviation analysis was performed using CalECG software (AMPS LLC). All analyses were performed by two blinded analysts. In case of doubt, a third analyst re-evaluated the ECG, so that consensus could be reached in all cases. ST-segment deviation was measured at the J point in each of the 12 ECG leads on the ambulance ECG and expressed in millimetres deviation. J point deviations from each individual ECG lead were totalled for a cumulative ST-segment deviation. A large myocardial area at risk was defined as a cumulative ST-segment deviation of >15 mm on the ambulance ECG, based on existing literature12.
ENDPOINTS
We assessed early myocardial reperfusion parameters and clinical outcomes. Early myocardial reperfusion parameters included the following angiographic markers: TIMI 3 flow, high thrombus burden (defined as TIMI thrombus grade ≥3), and myocardial blush grade 3. All parameters were reviewed both pre- and post-procedure. Furthermore, clinical outcomes including all-cause mortality, myocardial reinfarction, stent thrombosis, glycoprotein IIb/IIIa inhibitor bailout therapy, stroke, and urgent revascularisation were assessed between groups. All angiographic parameters were reviewed by an independent, blinded core laboratory. Moreover, all clinical events were adjudicated by an independent, blinded clinical event committee.
STATISTICAL METHODS
All statistical analyses were performed using the SPSS Statistics (version 26.0.0.1) software package for Windows (IBM). Patients were stratified into two groups according to the prehospital myocardial area at risk on the ambulance ECG (non-large: ≤15 mm, or large: >15 mm). Univariate logistic regression analysis was used to assess myocardial reperfusion parameters and clinical outcomes between groups. Results were reported as odds ratios (OR) with 95% confidence intervals (CI), and a p-value of <0.05 was considered statistically significant. In case of rare events (i.e., an expected count of less than five in each category), we used Fisher’s exact test to compute p-values.
The treatment effect of crushed compared to integral prasugrel loading dose administration was assessed by prehospital myocardial area at risk for all myocardial reperfusion endpoints and using logistic regression analysis for clinical outcomes. These results are provided as OR with 95% CI, with corresponding p-values for interaction.
Results
BASELINE CHARACTERISTICS AND PROCEDURAL DETAILS IN STEMI PATIENTS WITH PREHOSPITAL LARGE VERSUS NON-LARGE MYOCARDIAL AREA AT RISK ON THE AMBULANCE ECG
Out of 633 STEMI patients enrolled in the CompareCrush trial, 532 were eligible for ambulance ECG analysis, and 331/532 (62%) were identified as having a prehospital large myocardial area at risk. The baseline characteristics for patients with a prehospital large and non-large myocardial area at risk on the ambulance ECG are summarised in Table 1. The mean age at presentation was 61±12 years in patients with a prehospital large myocardial area at risk and 63±12 years in patients with a prehospital non-large myocardial area at risk (p=0.06). Female patients were equally represented in both groups (large: 24% vs non-large: 24%; p=0.83). A history of hypertension was numerically less common in patients with a prehospital large myocardial area at risk compared with patients with a prehospital non-large myocardial area at risk (35% vs 43%; p=0.07). Furthermore, incidences of other cardiovascular risk factors, including diabetes mellitus, dyslipidaemia, and smoking, were comparable between groups (large: 14% vs non-large: 17%; p=0.33; large: 25% vs non-large: 30%; p=0.28; and large: 46% vs non-large: 40%; p=0.21, respectively).
At first medical contact with the ambulance service, the median time since symptom onset was 60 (interquartile range [IQR] 34-134) minutes in the prehospital large myocardial area at risk group and 55 (IQR 23-116) minutes in the prehospital non-large myocardial area at risk group (p=0.11). After the initial ambulance assessment, the proportion of patients receiving a crushed prasugrel loading dose was comparable between groups (large: 52% vs non-large: 52%; p=0.91). During initial angiography, a thrombotic occlusion of the left anterior descending was significantly more common in patients with a prehospital large myocardial area at risk compared with patients with a prehospital non-large myocardial area at risk (46% vs 31%; p=0.001).
Table 1. Baseline and procedural characteristics.
| Large infarction N=331 | Non-large infarction N=201 | p-value | |
|---|---|---|---|
| Patient characteristics | |||
| Demographics | |||
| Age, years | 61±12 | 63±12 | 0.06 |
| Female | 78 (23.6) | 49 (24.4) | 0.83 |
| Caucasian | 301 (91.8) | 181 (91.4) | 0.89 |
| BMI, kg/m2 a | 27±4 | 28±4 | 0.21 |
| Cardiovascular risk factors | |||
| Hypertension | 113/325 (34.8) | 84/197 (42.6) | 0.07 |
| Diabetes mellitus | 44/325 (13.5) | 33/198 (16.7) | 0.33 |
| Dyslipidaemia | 79/313 (25.2) | 55/185 (29.7) | 0.28 |
| Smoking | 146/318 (45.9) | 78/194 (40.2) | 0.21 |
| Family history of cardiovascular disease | 123/313 (39.3) | 82/192 (42.7) | 0.45 |
| Medical history | |||
| Previous PCI | 25/330 (7.6) | 18 (9.0) | 0.57 |
| Previous myocardial infarction | 37/330 (11.2) | 27 (13.4) | 0.45 |
| Presentation | |||
| Symptom onset to first medical contact, mins | 60 [34-134] | 55 [23-116] | 0.11 |
| Crushed prasugrel | 173 (52.3) | 104 (51.7) | 0.91 |
| Procedural characteristics | |||
| Femoral access | 10/327 (3.1) | 6/192 (3.1) | 0.97 |
| Prasugrel loading dose to start of angiography, mins b | 44 [35-55] | 47 [37-59] | 0.045 |
| GPI administration | 43/331 (13.0) | 21/200 (10.5) | 0.39 |
| Clopidogrel maintenance | 5/331 (1.5) | 5/201 (2.5) | 0.42 |
| Culprit vessel | |||
| LAD | 152 (45.9) | 63 (31.3) | 0.001 |
| Cx | 131 (39.6) | 93 (46.3) | 0.13 |
| RCA | 42 (12.7) | 37 (18.4) | 0.07 |
| Thrombectomy | 69/327 (21.1) | 29/192 (15.1) | 0.09 |
| Total procedural time, mins c | 35 [25-50] | 36 [25-50] | 0.64 |
| ACT at sheath insertion, secs | 151 [131-175] | 147 [124-178] | 0.21 |
| ACT at sheath removal, secs | 229 [196-264] | 216 [170-263] | 0.028 |
| Data are presented as mean±standard deviation, n (%), n/N (%) or median [IQR]. a available in 208 vs 131 patients; b available in 325 vs 192 patients; c available in 324 vs 192 patients. ACT: activated clotting time; BMI: body mass index; Cx: circumflex artery; GPI: glycoprotein inhibitors; IQR: interquartile range; LAD: left anterior descending artery; PCI: percutaneous coronary intervention; RCA: right coronary artery | |||
MYOCARDIAL REPERFUSION MARKERS AND CLINICAL OUTCOMES IN STEMI PATIENTS WITH PREHOSPITAL LARGE VERSUS NON-LARGE MYOCARDIAL AREA AT RISK ON THE AMBULANCE ECG
Myocardial reperfusion endpoints and clinical outcomes are described in Table 2. Fully restored infarct-related vessel patency on initial angiography was present in 28% of patients with a prehospital large myocardial area at risk and in 41% of patients with a prehospital non-large myocardial area at risk (OR 0.56, 95% CI: 0.38-0.82; p=0.003). In line with this, a high thrombus burden in the IRA on initial angiography was more common in the prehospital large myocardial area at risk group compared with the prehospital non-large myocardial area at risk group (86% vs 77%, OR 1.80, 95% CI: 1.13-2.87; p=0.013). In contrast, pre-PCI myocardial blush grade 3 rates were numerically but not significantly lower in the prehospital large myocardial area at risk group (9% vs 12%, OR 0.67, 95% CI: 0.32-1.40; p=0.29). Postprocedural TIMI 3 flow in the IRA occurred less frequently in patients who presented with a prehospital large myocardial area at risk (86% vs 93%, OR 0.46, 95% CI: 0.24-0.88; p=0.019). However, the occurrence of postprocedural myocardial blush grade 3 was similar between groups (large: 55% vs non-large: 55%, OR 0.98, 95% CI: 0.61-1.57; p=0.93).
All-cause mortality rates at 1-year follow-up did not significantly differ between groups (large: 2.5% vs non-large: 1.6%, OR 1.59, 95% CI: 0.42-6.05; p=0.50). Moreover, other clinical outcomes at one year, including myocardial reinfarction, stent thrombosis, stroke, urgent revascularisation and major adverse cardiac and cerebrovascular events (MACCE), were comparable between groups.
Table 2. Reperfusion and clinical outcomes.
| Large infarction | Non-large infarction | Odds ratio [95% CI] | p-value | |
|---|---|---|---|---|
| Early myocardial reperfusion | ||||
| Pre-PCI | ||||
| TIMI 3 flow in the IRA | 87/308 (28.2) | 78/189 (41.3) | 0.56 [0.38-0.82] | 0.003 |
| High thrombus burden in the IRA | 263/307 (85.7) | 146/190 (76.8) | 1.80 [1.13-2.87] | 0.013 |
| Myocardial blush grade 3 | 18/210 (8.6) | 14/114 (12.3) | 0.67 [0.32-1.40] | 0.29 |
| Post-PCI | ||||
| TIMI 3 flow in the IRA | 262/306 (85.6) | 168/181 (92.8) | 0.46 [0.24-0.88] | 0.019 |
| High thrombus burden in the IRA | 4/307 (1.3) | 1/180 (0.6) | 2.36 [0.26-21.31] | 0.44 |
| Myocardial blush grade 3 | 104/191 (54.5) | 61/111 (55.0) | 0.98 [0.61-1.57] | 0.93 |
| Clinical outcomes at 30 days | ||||
| MACCE | 40 (12.3) | 20 (10.3) | 1.23 [0.70-2.18] | 0.47 |
| Clinical outcomes at 1 year | ||||
| Death | 8/324 (2.5) | 3/191 (1.6) | 1.59 [0.42-6.05] | 0.50 |
| Myocardial reinfarction | 11/324 (3.4) | 13/191 (6.8) | 0.48 [0.21-1.10] | 0.08 |
| Stent thrombosis | 4/324 (1.2) | 2/191 (1.0) | 1.18 [0.21-6.51] | 1.00 |
| Stroke | 3/324 (0.9) | 2/191 (1.0) | 0.88 [0.15-5.33] | 1.00 |
| Urgent revascularisation | 9/324 (2.8) | 9/191 (4.7) | 0.58 [0.23-1.48] | 0.25 |
| MACCE | 24/324 (7.4) | 18/191 (9.4) | 0.77 [0.41-1.46] | 0.42 |
| Data are presented as n/N (%) or n (%). CI: confidence interval; IRA: infarct-related artery; MACCE: major adverse cardiac and cerebrovascular events; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction | ||||
EFFICACY OF CRUSHED VERSUS INTEGRAL PRASUGREL IN STEMI PATIENTS WITH PREHOSPITAL LARGE AND NON-LARGE MYOCARDIAL AREA AT RISK ON THE AMBULANCE ECG
The interaction analyses for the early myocardial reperfusion endpoints according to randomised treatment group and prehospital myocardial area at risk are shown in Table 3 and Figure 1. The effect of crushed prasugrel compared with integral prasugrel on preprocedural TIMI 3 flow in the IRA was consistent in patients with a prehospital large myocardial area at risk (28% vs 29%, OR 0.93, 95% CI: 0.56-1.52) and patients with a prehospital non-large myocardial area at risk (40% vs 42%, OR 0.93, 95% CI: 0.52-1.66; pinteraction=1.00). Moreover, the effect of crushing prasugrel loading dose tablets on other preprocedural angiographic parameters was consistent across patients with prehospital large and non-large myocardial areas at risk.
In terms of post-PCI angiographic outcomes, no differential treatment effect of crushed versus integral prasugrel administration in terms of myocardial blush grade was observed between the prehospital large and non-large myocardial areas at risk groups. However, administration of crushed prasugrel significantly increased the rate of postprocedural TIMI 3 flow in the IRA in patients with a prehospital large myocardial area at risk (crushed: 92% vs integral: 79%, OR 3.00, 95% CI: 1.50-6.00), whereas crushed prasugrel administration in patients with a prehospital non-large myocardial area at risk was not associated with a lower incidence of postprocedural TIMI 3 flow in the IRA (crushed: 91% vs integral: 95%, OR 0.47, 95 % CI: 0.14-1.57; pinteraction=0.009) (Central illustration).
Table 3. Interaction analysis between randomisation sequence and large versus non-large infarction area.
| Total no. of patients | Crushed prasugrel | Integral prasugrel | Odds ratio [95% CI] | p-value | |
|---|---|---|---|---|---|
| Pre-PCI | |||||
| TIMI 3 flow in the IRA | |||||
| Large infarction | 308 | 44/160 (27.5) | 43/148 (29.1) | 0.93 [0.56-1.52] | 1.00 |
| Non-large infarction | 189 | 40/99 (40.4) | 38/90 (42.2) | 0.93 [0.52-1.66] | |
| High thrombus burden in the IRA | |||||
| Large infarction | 307 | 133/159 (83.6) | 130/148 (87.8) | 0.71 [0.37-1.35] | 0.13 |
| Non-large infarction | 190 | 80/100 (80.0) | 66/90 (73.3) | 1.46 [0.74-2.86] | |
| Myocardial blush grade 3 | |||||
| Large infarction | 210 | 9/107 (8.4) | 9/103 (8.7) | 0.96 [0.37-2.52] | 0.52 |
| Non-large infarction | 114 | 6/62 (9.7) | 8/52 (15.4) | 0.59 [0.19-1.82] | |
| Post-PCI | |||||
| TIMI 3 flow in the IRA | |||||
| Large infarction | 306 | 146/159 (91.8) | 116/147 (78.9) | 3.00 [1.50-6.00] | 0.009 |
| Non-large infarction | 181 | 86/95 (90.5) | 82/86 (95.3) | 0.47 [0.14-1.57] | |
| High thrombus burden in the IRA | |||||
| Large infarction | 307 | 2/160 (1.3) | 2/147 (1.4) | 0.92 [0.13-6.60] | - |
| Non-large infarction | 179 | 1/94 (1.1) | 0/85 (0.0) | - | |
| Myocardial blush grade 3 | |||||
| Large infarction | 191 | 54/92 (58.7) | 50/99 (50.5) | 1.39 [0.79-2.47] | 0.26 |
| Non-large infarction | 111 | 31/59 (52.5) | 30/52 (57.7) | 0.81 [0.38-1.72] | |
| Data are presented as n/N (%). CI: confidence interval; IRA: infarct-related artery; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction | |||||
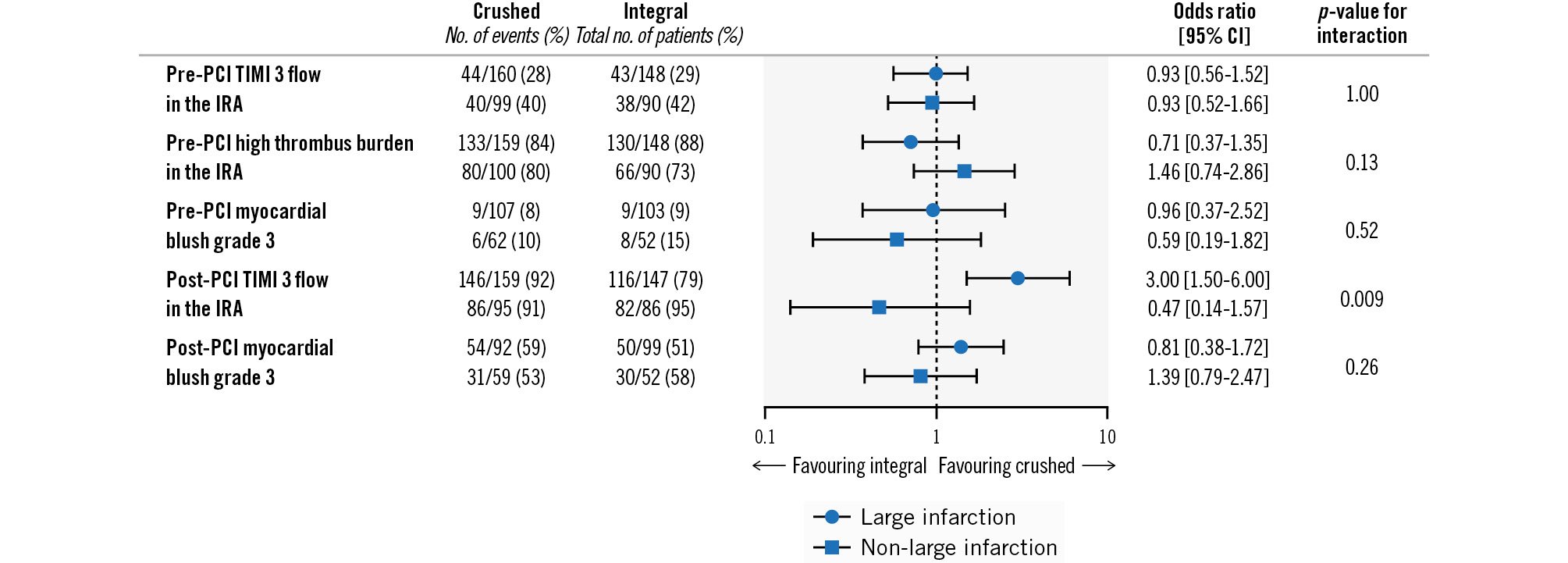
Figure 1. Angiographic endpoints in STEMI patients with prehospital large and non-large infarction area at risk on the ambulance ECG who were treated with crushed versus integral tablets of prasugrel loading dose. CI: confidence interval; ECG: electrocardiography; IRA: infarct-related artery; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction
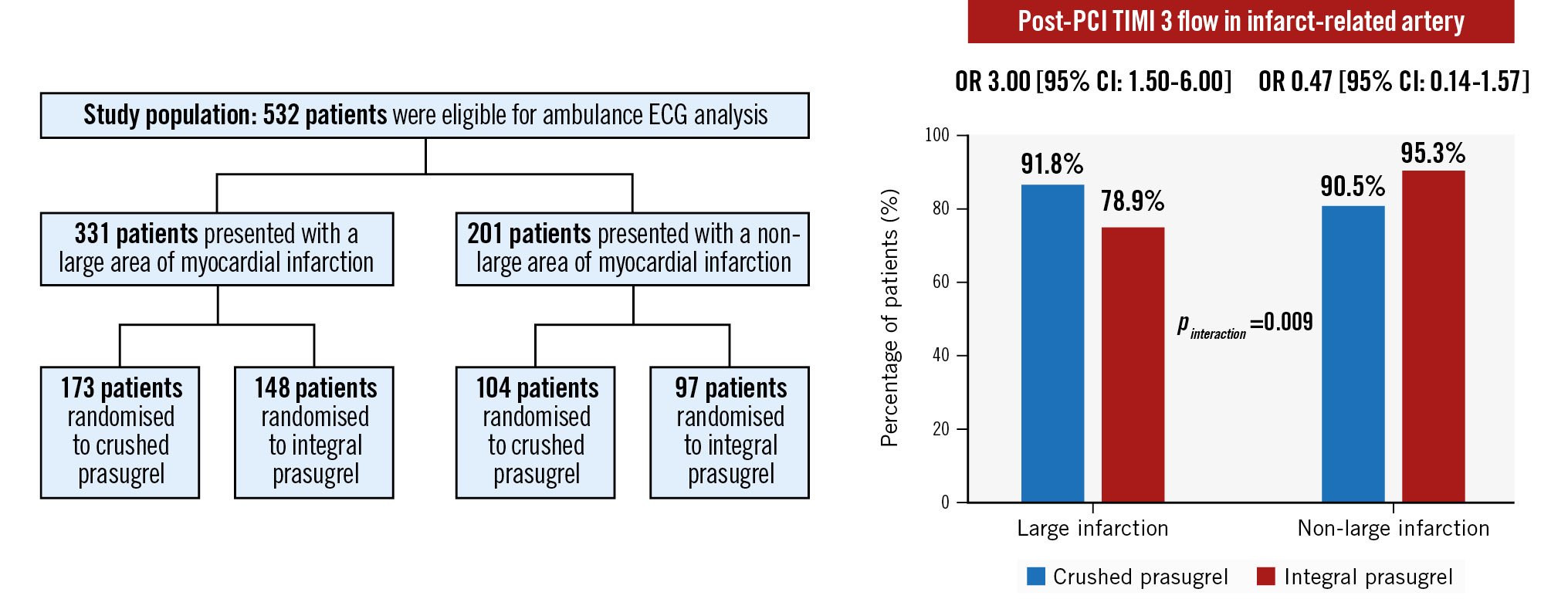
Central illustration. Impact of crushed prasugrel on post-PCI TIMI 3 flow in infarct-related arteries. CI: confidence interval; ECG: electrocardiography; OR: odds ratio; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction
Discussion
The results of this prespecified CompareCrush substudy can be summarised as follows: 1) Over 60% of all STEMI patients presented with a prehospital large myocardial area at risk on the ambulance ECG, and these patients exhibited consistently worse pre- and postprocedural angiographic outcomes in comparison with STEMI patients with a prehospital non-large myocardial area at risk. 2) We found a significant interaction between randomised prasugrel treatment and prehospital myocardial area at risk on the ambulance ECG, indicating that administration of crushed compared with integral tablets of prasugrel loading dose leads to a greater improvement in postprocedural TIMI 3 flow in patients with a prehospital large myocardial area at risk, compared to patients with a prehospital non-large myocardial area at risk.
Suboptimal epicardial flow pre-PCI has been previously associated with lower procedural success rates and an increased final infarct size131415. The final infarct size represents an important determinant for survival in STEMI patients undergoing primary PCI, and every 5% increase in the final infarct size on CMR has been associated with a 19% relative risk increase for one-year mortality (per 5% infarct size increase: OR 1.19, 95% CI: 1.18-1.20; p<0.0001)716. The magnitude of the final infarct size is largely dependent on the duration of ischaemia. Therefore, most of the pharmacological, mechanical and logistical innovations that have taken place in the last eighty years were primarily aimed at reducing the ischaemic time of the infarct-related myocardial tissue51617. These innovations have substantially contributed to fast epicardial reperfusion and shorter total ischaemic times. However, despite timely and successful revascularisation, microvascular obstruction in the downstream area of the infarct-related artery still occurs in up to 10% of patients and significantly contributes to the final infarct size418. Therefore, therapies aimed at further reducing final myocardial infarct size remain warranted.
The present report showed a significant interaction between the randomised prasugrel treatment allocation and the prehospital myocardial area at risk on the ambulance ECG, indicating that crushed prasugrel administration extends a greater benefit in terms of postprocedural TIMI 3 flow in the IRA in patients with a prehospital large myocardial area at risk, compared to patients with a non-large myocardial area at risk, on the ambulance ECG. Administration of a crushed prasugrel loading dose in STEMI patients facilitates early and strong platelet inhibition, which may have contributed to the observed procedural success1019. Early and potent platelet inhibition has been shown to destabilise existing thrombus material and to attenuate continuing thromboÂgenesis on the circumference of the culprit lesion, thereby allowing for easier recanalisation and revascularisation of the infarct-related artery2021222324. Moreover, early and potent platelet inhibition may reduce the extent of distal embolisation, platelet-leukocyte microplugging, and inflammation in the myocardial microcirculation342225. Current literature does not provide a clear explanation as to why a more potent platelet inhibition would only benefit patients presenting with a large area of myocardial infarction. However, hypothetically, a more potent platelet inhibition might have more benefit on a larger absolute microvascular volume which is, in turn, more likely to demonstrate a statistically significant effect. Interestingly, the randomised CompareCrush trial – which was primarily designed to investigate the hypothesis that early, strong platelet inhibition could improve surrogate markers of early myocardial reperfusion in STEMI patients undergoing primary PCI – failed to show any beneficial effect associated with the administration of a crushed prasugrel loading dose11. However, as observed in other investigations, it cannot be ruled out that the short time interval between taking the study medication and the start of primary PCI (median of only 45 minutes) was responsible for the lack of significance in this trial. Of note, we observed a significant difference in the time interval between prasugrel administration and primary PCI which could have had an impact on the direct comparison of the large and non-large infarct groups. Moreover, the considerable proportion of patients who exhibited a prehospital non-large myocardial area at risk (almost 40% of the cohort) could have diluted a potential benefit of crushed prasugrel administration in the overall study. Whether administering a prehospital crushed prasugrel loading dose in STEMI patients presenting with a prehospital large myocardial area at risk on the ambulance ECG truly improves angiographic success remains to be determined in a large adequately powered trial.
In the present substudy, we used cumulative ST-segment deviation on the ambulance ECG as a stratification variable to identify prehospital large and non-large myocardial area at risk patients. Previous trials have linked extensive cumulative ST-segment deviation on the ambulance ECG to higher peak concentrations of creatinine kinase, indicating a large infarct size in STEMI patients scheduled for primary PCI6. Moreover, extensive cumulative ST-segment deviation has been identified as an independent predictor of both 30-day and long-term mortality626. As the CompareCrush trial did not include prespecified CMR or other imaging modalities to assess the final infarct size, the use of cumulative ST-segment deviation as a stratification variable for expected myocardial infarction size allowed us to explore the potential benefit of crushed prasugrel administration in a high-risk subgroup of STEMI patients. However, supporting CMR data are needed to further establish the effect of crushed prasugrel loading dose administration on the final myocardial infarct size in STEMI patients presenting with a prehospital large myocardial area at risk on the ambulance ECG2728.
Limitations
The results of this substudy should be considered in the light of several limitations. First, this was a post hoc analysis performed in the CompareCrush cohort, which was not adequately powered to assess differences in terms of clinical outcomes, and therefore, procedural outcomes should be interpreted as hypothesis-generating only. Additional research is required to investigate how an increase in postprocedural TIMI 3 flow among patients translates into clinical outcomes after administration of crushed prasugrel. Second, despite considerable efforts to minimise the extent of missing data, ECGs were missing for approximately 16% of the patients. Third, cardiac enzyme progression and CMR imaging modalities are commonly used to identify infarct size in STEMI patients. However, given the acute randomisation phase of this study, cumulative ST-segment deviation was used as a predictor for infarct size, which has been used as a stratification tool in several clinical trials. Yet, it must be noted that the cutoff value of 15 mm for cumulative ST-segment deviation is not a validated marker of a large area of myocardium at risk. Follow-up with cardiac enzyme progression and CMR modalities would have provided a better understanding of the reduction of infarct size6122930. Third, this substudy was not sufficiently powered to assess the heterogeneity of clinical outcomes. Studies with a higher number of patients are required to adequately investigate if the higher rate of post-PCI TIMI 3 flow translates into a lower rate of MACCE in patients with a large area of myocardial infarction. Fourth, data regarding the use of medication on admission and during the PCI procedure were not collected, which could otherÂwise have added to the interpretation of our study results.
Conclusions
In this prespecified substudy, we saw a significant effect of crushed over integral prasugrel loading dose administration in terms of procedural success in STEMI patients undergoing primary PCI with a prehospital large versus non-large myocardial area at risk on the ambulance ECG. These findings suggest that administration of crushed tablets of prasugrel loading dose facilitates postprocedural TIMI 3 flow in patients with a prehospital large myocardial area at risk and, therefore, represents an attractive, fast and cost-effective strategy compared to the administration of integral tablets. However, data from this substudy were derived from a trial that demonstrated negative primary results, and, therefore, these findings should be considered hypothesis-generating. Dedicated and adequately powered clinical trials are warranted to confirm these findings.
Impact on daily practice
What is known: Increased infarction size is associated with poor postprocedural reperfusion success and mortality. The introduction of prehospital pharmacological treatment regimens have led to a reduction in myocardial infarction burden. The randomised CompareCrush trial suggested a reduction in early platelet reactivity during primary percutaneous coronary intervention when crushing tablets of prasugrel loading dose, but it remains unclear whether early, enhanced platelet inhibition could benefit the subgroup of high-risk ST-segment elevation myocardial infarction (STEMI) patients presenting with a large myocardial infarction area on ambulance electrocardiography (ECG).
What is new: In patients presenting with STEMI and a prehospital large myocardial area at risk on ambulance ECG undergoing primary percutaneous coronary intervention, administration of a crushed prasugrel loading dose was associated with a higher frequency of postprocedural Thrombolysis in Myocardial Infarction grade 3 flow (odds ratio [OR] 3.00, 95% confidence interval [CI]: 1.50-6.00) compared with integral prasugrel administration (OR 0.47, 95% CI: 0.14-1.57; pinteraction=0.009).
What is next: Whether prehospital administration of a crushed prasugrel loading dose in STEMI patients presenting with a large myocardial area at risk on the ambulance ECG truly improves prognosis remains to be determined in a large, adequately powered trial.
Acknowledgements
We would like to extend our gratitude to the regional ambulance service “AmbulanceZorg Rotterdam-Rijnmond”, in particular M. Biekart, and the catheterisation laboratories and cardiac care units of Maasstad Hospital and Erasmus Medical Center. Additionally, we would like to thank the DSMB: F.W.A. Verheugt, J.G.P. Tijssen, and M. Voskuil; the CEC: K.T. Koch and M. Meuwissen; and the STEMI adjudication committee: F. Nijhoff and M. Grundeken. Furthermore, we thank A. Ruiter, J. Rijssemus, R. van Dam and C. Vliet for clinical data acquisition and J. Uiters from Medwave, M. Vaglio and F. Badilini from AMPS LLC, and L.L.P.J. Kuijten for their support.
Funding
The CompareCrush trial was funded by Maasstad research B.V. (Rotterdam, the Netherlands), which received unrestricted grants from Daiichi Sankyo and Shanghai MicroPort Medical. The funding companies were not involved in the conduct of the trial, the analysis of the data, or the drafting of the manuscripts.
Conflict of interest statement
D.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura; he also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. D. Alexopoulos declares that he has received consulting fees or honoraria from AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Chiesi Hellas, Medtronic, and Pfizer. G. Montalescot reports research or educational grants to the institution or consulting/lecture fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, CellProthera, Europa, Servier Iris, Novartis, Medtronic, MSD, Pfizer, Quantum Genomics, and Sanofi-Aventis. N.M. Van Mieghem has received institutional research grant support from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Teleflex, PulseCath BV, and Daiichi Sankyo. P.C. Smits declares that he has received research grants from Daiichi Sankyo and Shanghai MicroPort. G.J. Vlachojannis has received consulting fees from AstraZeneca; and research grants from Daiichi Sankyo and Shanghai MicroPort. The other authors have no conflicts of interest to declare.

