Abstract
Background: Contrast-induced acute kidney injury (CI-AKI) is prognostically relevant in invasive cardiological and radiological procedures. The administration of sodium bicarbonate has controversial effects. It has been hypothesised that bicarbonate is ineffective when unable to achieve adequate urine alkalinisation.
Aims: We tested the hypothesis that alkaline urine status with oral or intravenous (i.v.) bicarbonate on top of hydration alone prevents CI-AKI.
Methods: In a prospective, randomised, parallel-group, open-label trial, we compared 1) saline hydration alone (n=81); 2) i.v. bicarbonate (n=82); and 3) oral bicarbonate (n=78), in patients with chronic kidney disease (CKD) scheduled for the intra-arterial administration of contrast medium. The primary endpoint was the incidence of CI-AKI according to alkaline urine status achieved immediately before angiography. Secondary endpoints were the mean change of urine pH up to the time of angiography and the incidence of CI-AKI in the three groups.
Results: The incidence of CI-AKI was not significantly different in the three treatment arms (20% in the hydration group, 21% in the oral bicarbonate group and 22% in the i.v. bicarbonate group; p=0.94). Patients achieving a pH >6 before angiography (n=145) had a significantly lower incidence of CI-AKI compared with the others (n=96; odds ratio [OR] 0.48, 95% confidence interval [CI]: 0.25-0.90; p=0.023, primary study hypothesis). The proportion of patients achieving a pH >6 was higher in the i.v. and oral bicarbonate groups compared with hydration alone.
Conclusions: Urinary pH before administration of contrast medium is an inverse correlate of CI-AKI incidence, and bicarbonate is superior to hydration alone in achieving urinary alkalinisation. Since, however, bicarbonate did not reduce the incidence of CI-AKI, we conclude that urinary pH is a marker and not a mediator of CI-AKI (ClinicalTrials.gov: NCT02980003).
Introduction
A post-procedural renal injury is a recognised adverse effect of iodinated contrast media and accounts for increased morbidity and mortality after angiography and percutaneous coronary intervention (PCI)1. The occurrence of contrast-induced acute kidney injury (CI-AKI) ranges from 2% in low-risk to 50% in high-risk patients2. The most important risk factors for CI-AKI development are pre-existing renal failure, diabetes, age, volume and type of contrast medium2.
Treatment of CI-AKI is exclusively supportive, and particularly recommended in patients with chronic kidney disease (CKD, classified as mild, moderate or severe according to the estimated glomerular filtration rate [eGFR]). Among approaches tested for CI-AKI prevention3, hydration is widely accepted and is today the only strategy advised by international guidelines (class of recommendation I, level of evidence A)45. The efficacy of sodium bicarbonate (bicarbonate) in preventing CI-AKI has been extensively tested, with the rationale that urine alkalinisation suppresses the formation of free radicals. Studies on bicarbonate, however, have been controversial, with effects spanning from beneficial to neutral2.
We hypothesised that urine alkalinisation is the real mediator of CI-AKI prevention, and that bicarbonate, delivered either intravenously (i.v.) or orally, to achieve urine alkalinisation, is an effective preventive strategy. Specifically, we tested three sequential hypotheses: (1) that urine alkaline status (pH >6.0) is significantly associated with a lesser incidence of CI-AKI; (2) that bicarbonate (either i.v. or oral) achieves a better urine alkalinisation (pH >6.0) than hydration alone; and (3) that bicarbonate (i.v., oral or combined), by determining a better urinary alkalinisation, would prevent CI-AKI. We tested this in a randomised, open-label, prospective, multicentre, three-arm study.
Methods
The PrevenTion of contrast-inducEd nephropAThy with urinE alkalinisation (TEATE) study was a not-for-profit, prospective, randomised, parallel-group, open-label trial, conducted in three centres: the SS. Annunziata University Hospital in Chieti (promoting centre), the Misericordia Hospital in Grosseto, and the Mazzoni Hospital in Ascoli Piceno, all in Italy. The study aimed at establishing the role of urine pH and of urine alkalinisation (here defined as the achievement of a urinary pH >6.0 before angiography) for CI-AKI prevention comparing different strategies in patients with moderate or severe CKD.
The trial was approved by the three ethics committees of the recruiting centres and by the Italian Drug Agency (Agenzia Italiana del Farmaco, AIFA). The study protocol was registered on the ClinicalTrials.gov website with the NCT02980003 identification number. The trial design, with further details of methods used, has been published2. At variance from the original study design, because of difficulties in blinding the administration of oral bicarbonate, the actual study design was open-label. Participating patients were covered by an insurance.
Inclusion and exclusion criteria
We considered for enrolment consecutive patients scheduled for coronary angiography and/or angioplasty. Patients were considered eligible if they fulfilled the following criteria:
- age ≥18 years;
- eGFR <60 mL/min/1.73 m2, but >15 mL/min/1.73 m2 (Modification of Diet in Renal Disease [MDRD] equation).
Patients were excluded for the occurrence of any of the following:
- acute renal insufficiency;
- emergency catheterisation (e.g., ST-elevation myocardial infarction [STEMI] patients) preventing the possibility of pre-treatment;
- a history of adverse reactions to contrast media;
- use of potentially nephrotoxic drugs (non-steroidal anti-inflammatory drugs, aminoglycosides, sulphonamides, cyclosporine, tacrolimus, methotrexate or platinum complexes) from 48 hours before to 24 hours after the procedure, but allowing drugs deemed essential for cardiovascular therapy (diuretics, acetylsalicylic acid, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers or aliskiren);
- pulmonary oedema;
- multiple myeloma and other monoclonal gammopathies;
- factors predisposing to kidney injury: diarrhoea, vomiting, dehydration or bleeding;
- exposure to contrast media within 7 days before the procedure;
- pregnancy;
- hypersensitivity to the active substance or to any of the excipients;
- metabolic or respiratory alkalosis, particularly if hypochloraemic (vomiting, gastrointestinal losses, diuretic therapy);
- hypocalcaemia;
- use of N-acetyl cysteine, theophylline, dopamine, fenoldopam, mannitol, citrate or bicarbonate within 48 hours before coronary angiography;
- chronic and/or acute therapy with corticosteroid, quinidine, ephedrine and pseudoephedrine;
- urinary tract infections.
All recruited patients provided written informed consent.
Randomisation, interventions and kidney-function measures
After enrolment, patients were randomised (in a 1:1:1 ratio) into three groups: 1) hydration alone (control group); 2) hydration plus i.v. bicarbonate; 3) hydration plus oral bicarbonate (Figure 1). The tested prevention protocols were:
- Hydration alone (control group): patients were to start hydration with isotonic saline 6 hours before angiography and continue for 12 hours after the procedure. The infusion rate was to be 1 mL/kg/h (reduced to 0.5 mL/kg/h in the presence of an ejection fraction <35% or New York Heart Association [NYHA] Functional Class III or IV). During the hour before angiography the infusion rate was to be increased to 3 mL/kg/h to allow the infusion of an equal volume of fluids in all three arms of the protocol.
- Intravenous sodium bicarbonate: patients were to start hydration 6 hours before angiography; in the first 5 hours they were to receive isotonic saline at 1 mL/kg/h (reduced to 0.5 mL/kg/h if with an ejection fraction <35% or NYHA Functional Class III or IV). Then, a solution of 1.4% sodium bicarbonate (167 mEq/L; 334 mOsm/L) was to be infused: the initial i.v. bolus was to be 3 mL/kg/h for 1 hour immediately before contrast medium injection; following this, patients were to receive the same fluid solution at a rate of 1 mL/kg/h (reduced to 0.5 mL/kg/h if with an ejection fraction <35% or NYHA Functional Class III or IV) during the exposure to contrast and for 6 hours after the procedure. Later, patients were to resume hydration with isotonic saline (1 mL/kg/h; reduced to 0.5 mL/kg/h if with an ejection fraction <35% or NYHA Functional Class II or III) for a further 6 hours.
- Oral sodium bicarbonate: patients were to start hydration with isotonic saline with the same infusion rate and timing of the control group. One hour before the angiography and three hours after, patients were to receive oral sodium bicarbonate at the dose of 4 g (47.6 mEq) dissolved in 60 mL of water. The drug was to be weighed with a high-precision tuning fork electronic balance (mod. AJH-CEN 620 series, Shinko Denshi) with a sensitivity of ±0.1 mg and placed in a labelled, sterile, plastic container. The label reported the lot number, expiry date of the sodium bicarbonate lot, the signature of the pharmacist carrying out the weighing process, a serial number to identify the sample and the patient identification number. The documentation was stored in the laboratory of Galenic Preparations, Pharmacy Division, of the individual hospitals involved in the trial.
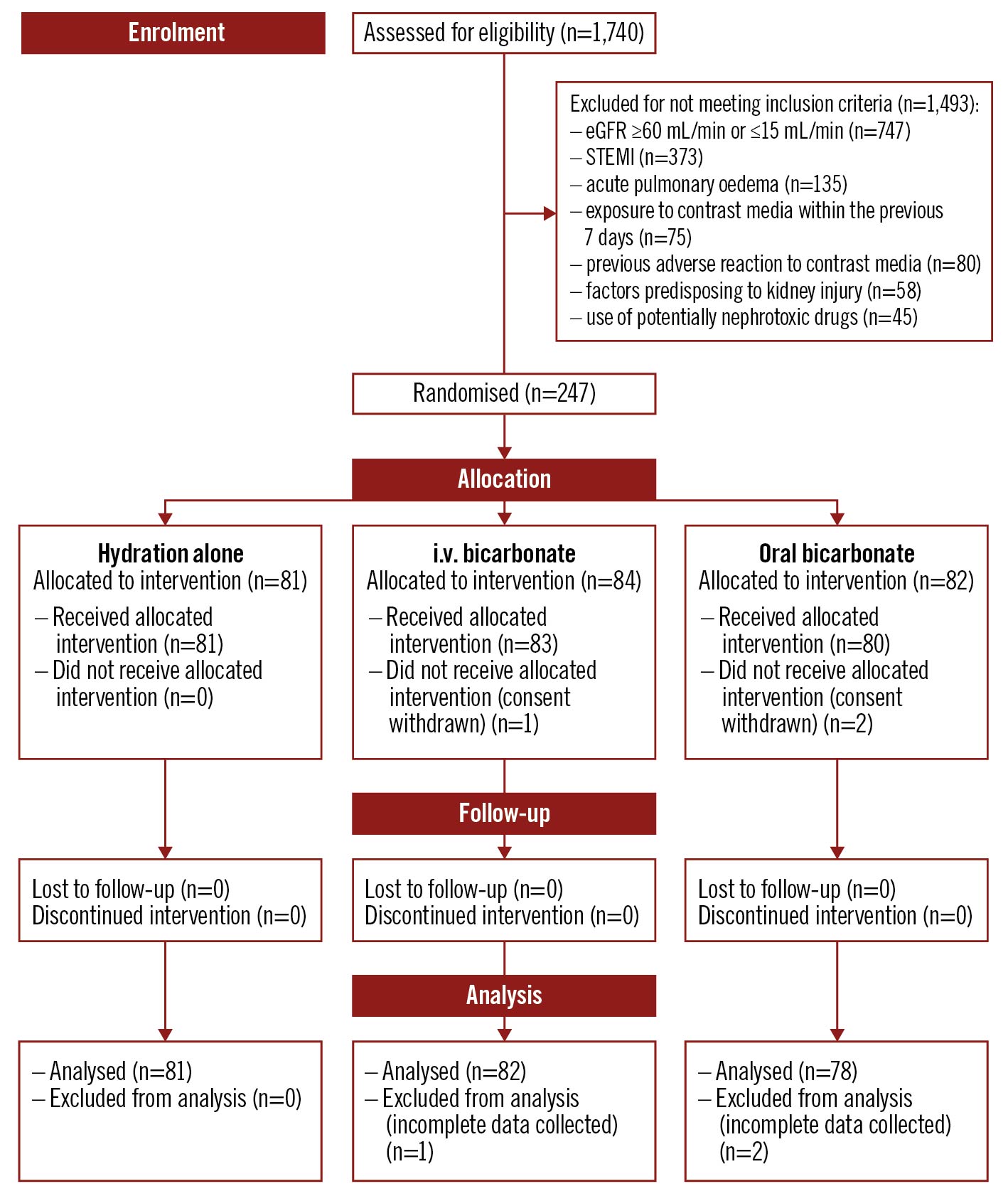
Figure 1. Patient disposition – TEATE trial CONSORT flow diagram. Patients scheduled for coronary angiography and/or angioplasty who met eligibility criteria were randomly allocated in a 1:1:1 ratio into 3 groups: hydration alone (control group), hydration with i.v. sodium bicarbonate, and hydration with oral sodium bicarbonate.
The allocation of patients to each group was based on a computer-generated randomisation list produced by the statistician (MDN), independent of the physicians enrolling patients and assigning them to interventions, with a probability of 1/3 per group, as previously detailed2. The physicians enrolling patients and assigning them to interventions were the staff doctors in each of the three participating centres.
The rate of any infusion was to be reduced in patients developing signs or symptoms of pulmonary congestion, and special precautions were taken in patients with dyspnoea for suspected pulmonary oedema, congestive heart failure, severe kidney disease (e.g., oliguria), severe liver disease (e.g., ascites, cirrhosis), high sodium levels, swollen ankles/legs/feet due to water retention (peripheral oedema). Electrolytes (Na, K, Ca) were checked and corrected, if necessary, before and after the procedure. Patients were all informed of possible side effects, as indicated in the patient information sheet specifically provided2. All additional infusions were strongly discouraged and initiated only if indicated for other reasons.
Demographic data, current medications and medical history were recorded at baseline on a specific case report form (CRF). At the end of the study, the CRF were sent to the Chieti SS. Annunziata Hospital centre, where the data were analysed.
Using an electronic pH meter, we determined urine pH in all patients at (1) hospital admission; (2) immediately before coronary angiography; and (3) 24-48 hours after the procedure. At admission and 24-48 hours after angiography, we evaluated 1) serum creatinine; 2) serum electrolytes; 3) cystatin C, an alternative marker of renal function, probably more reliable than creatinine in elderly (>75 years old) patients. Cystatin C is a promising, easily measurable marker to estimate GFR with a higher diagnostic yield than serum creatinine and an excellent accuracy for the early diagnosis of CI-AKI at 24 and 48 hours6; and 4) creatinine clearance (CrCl), indirectly estimating the GFR (eGFR) according to the MDRD formula7. For each patient, the type and amount of contrast medium administered during angiography were precisely recorded. Only iso-osmolar or hypo-osmolar contrast media were allowed, and namely: iobitridol (Xenetix 350; Guerbet); iopromide (Ultravist 300, Ultravist 370; Bayer); iomeprol (Iomeron 300, Iomeron 350; Bracco); and iodixanol (Visipaque 270, Visipaque 320; GE Healthcare).
According to the degree of urine alkalinisation achieved immediately before coronary angiography, two groups were formed, using a cut-off pH of 6.0 (≤6.0 vs >6.0).
Recruitment started in January 2015 and ended in December 2019, within the expected 60 months of recruitment period.
Endpoint definitions
Urinary alkalinisation was conventionally defined as reaching urinary pH ≥6 immediately before the administration of contrast medium (the terms “alkalinisation”, “alkaline” and “acidic” are used in agreement here with most of the related medical literature, not in strictly physical chemistry terms). CI-AKI was defined as a >25% increase in serum creatinine concentration and/or a >25% decrease in eGFR and/or an increase >10% of serum cystatin C concentration and/or a >44 mmol/L (0.5 mg/dL) absolute increase in serum creatinine from baseline within 24-48 hours after administration of the contrast media, according to Markota et al8 and Quintavalle et al9.
Primary endpoint
The primary study hypothesis was that the incidence of CI-AKI, according to the above definition, would be significantly different in patients achieving “adequate” urine alkaline status (pH ≥6.0) compared with patients not achieving it. According to the degree of urine alkaline status immediately before coronary angiography, two groups were formed, using the cut-off pH of 6.0 (≤6.0 vs >6.0). The primary endpoint was defined as the incidence of CI-AKI according to the achievement (yes/no) of “adequate” urine alkaline status immediately before angiography. This endpoint served for the sample size estimate.
Secondary endpoints
We set two secondary – necessarily exploratory – endpoints: (1) the mean change of urine pH from hospital admission to the time of angiography, to compare the different alkalinising capacity of the 3 strategies; (2) the incidence of CI-AKI in the 3 study arms. Further exploratory endpoints were the incidence of CI-AKI and the proportion of patients achieving urine alkalinisation in a direct comparison between oral and i.v. bicarbonate groups.
Statistical considerations and methods
Based on previous literature, we anticipated that 20% of patients would develop CI-AKI in the group with a preprocedural urinary pH ≤6.0 vs 5% in the group achieving adequate urine alkaline status (pH >6.0)8. Assuming a 2-sided type I error rate of 5% and a power of 90%, we calculated that a sample size of 114 patients for each group would be required to detect a significant reduction in the proportion of patients developing CI-AKI. Therefore, we conservatively hypothesised the following pre-angiography alkalinisation rate in the 3 groups: 10% for the control group and 70% for the other two groups. We also hypothesised a dropout rate of 1%. A minimum of 80 patients in each group (control, i.v. bicarbonate and oral bicarbonate), totalling 240 patients, were therefore enrolled. Sample size calculation and details of the randomisation procedure have been previously reported2.
Baseline characteristics summary statistics are presented as mean±standard deviation (SD) for parametric, and as median and interquartile range (IQR) for non-parametric distributions, as appropriate. Differences in patients’ characteristics between the two groups of urine alkaline status were tested by the Student’s t-test for unpaired data and the Pearson’s chi-square test for continuous and categorical variables, respectively. A logistic regression model was applied to determine the predictive characteristics of CI‐AKI. Results were expressed as the odds ratio (OR) and the corresponding 95% confidence interval (95% CI). For the primary endpoint, patients were also stratified according to preprocedural urine pH (≤6.0 and >6.0) after the initial treatment. Secondary analyses evaluated results in the 3 groups (control; i.v. bicarbonate; oral bicarbonate). Differences in patients’ characteristics across the 3 groups were tested by analysis of variance (ANOVA) or the Kruskal–Wallis H test (for continuous parametric or non-parametric variables, respectively) and the Pearson’s chi-square test for categorical variables. All statistical analyses were performed using the R Statistical Software (version 3.5.3; R Foundation for Statistical Computing). All tests were two-tailed, and a p-value <0.05 was considered indicative of a statistically significant association only after applying the Bonferroni post hoc correction (threshold set at p<0.0167).
Results
Baseline characteristics
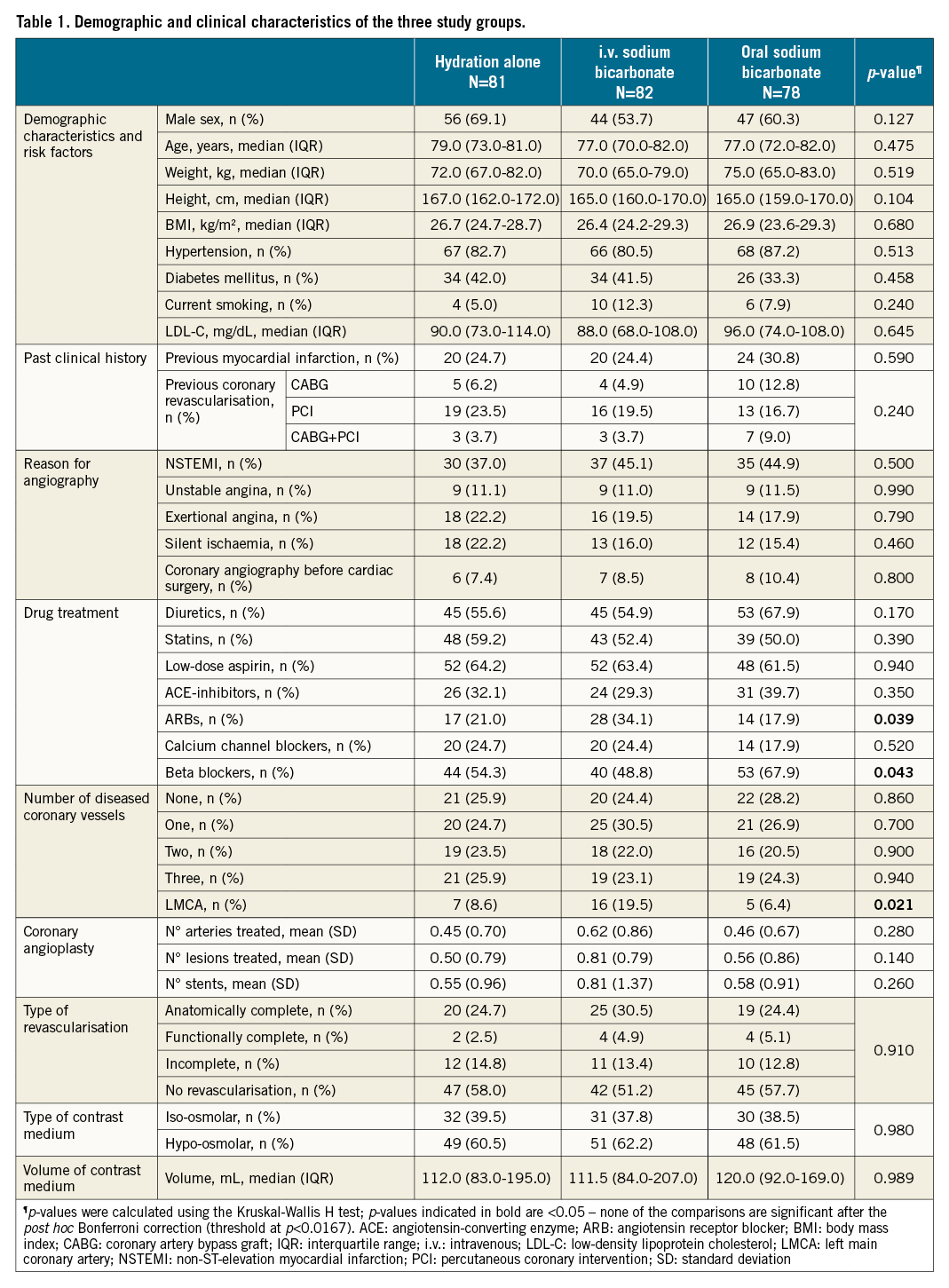
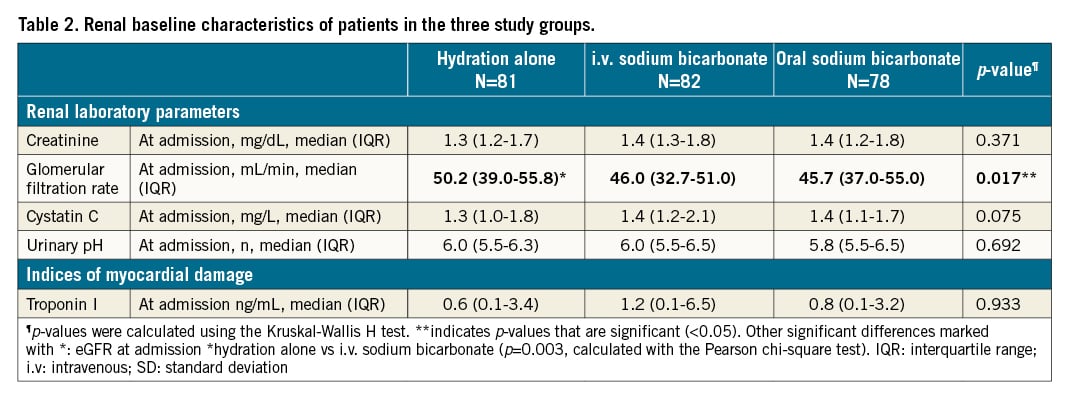
From May 2015 to May 2020, 1,740 patients were screened in the SS. Annunziata Hospital of Chieti, the Misericordia Hospital of Grosseto and the Mazzoni Hospital. Of these patients, 1,493 were excluded due to exclusion criteria, resulting in a total of 247 patients who were scheduled to receive the pharmacological interventions required by the study protocol. Of these, 81, 83, and 80 patients actually received the assigned treatments, and 81 receiving hydration alone, 82 receiving i.v. bicarbonate and 78 receiving oral bicarbonate were analysable (Figure 1, Table 1). The median age of the patients was 79 in the hydration group and 77 in both the bicarbonate groups. The population was homogeneous with regards to other baseline characteristics including medical history, laboratory parameters, and drug therapy. Minor differences occasionally occurring across the 3 groups investigated were not significant after the Bonferroni post hoc correction for multiple comparisons (Table 1). On admission, the mean eGFR value, calculated with the 4-factor MDRD formula, was 50.2 mL/min/1.73 m2 in the hydration group, 46 mL/min/1.73 m2 in the i.v. bicarbonate group and 45.7 in the oral bicarbonate group, with a formal statistically significant difference (p=0.017). Urinary pH was 6.0 in the hydration group, 6.0 in the i.v. bicarbonate group and 5.8 in the oral bicarbonate group, with no statistical difference (p=0.69) (Table 2).
Procedural characteristics
Patients undergoing coronary angiography alone were 58% in the hydration group, 51% in the i.v. bicarbonate group and 58% in the oral bicarbonate groups (Table 1). The median volume of contrast medium used was 112 mL in the hydration group, 112 mL in the i.v. bicarbonate group, and 120 mL in the oral bicarbonate group. The contrast medium used was hypo-osmolar in 61% of cases in the hydration group, in 62% in the i.v. bicarbonate group, and in 62% of cases in the oral bicarbonate group; in the remaining cases, an iso-osmolar contrast medium was used. The mean volume of i.v. fluids administered was the same in the 3 arms. The population in the 3 study groups was homogeneous for the other reported procedural characteristics (Table 1). A comparison of the study population stratified by baseline urinary pH is provided in Table 2, also showing the baseline comparability of the populations assigned to hydration alone or to hydration plus bicarbonate, either i.v. or oral.
Primary endpoint
Patients who had a urinary pH >6 (per protocol alkaline status) before angiography had a significantly lower incidence of CI-AKI compared with patients who immediately before the procedure maintained a urinary pH ≤6 (Table 3, Figure 2). The percentage of CI-AKI was 46% in patients with a preprocedural urinary pH >6; and 54% in patients with a preprocedural urinary pH ≤6 (OR 0.48, 95% CI: 0.25-0.90; p=0.023).
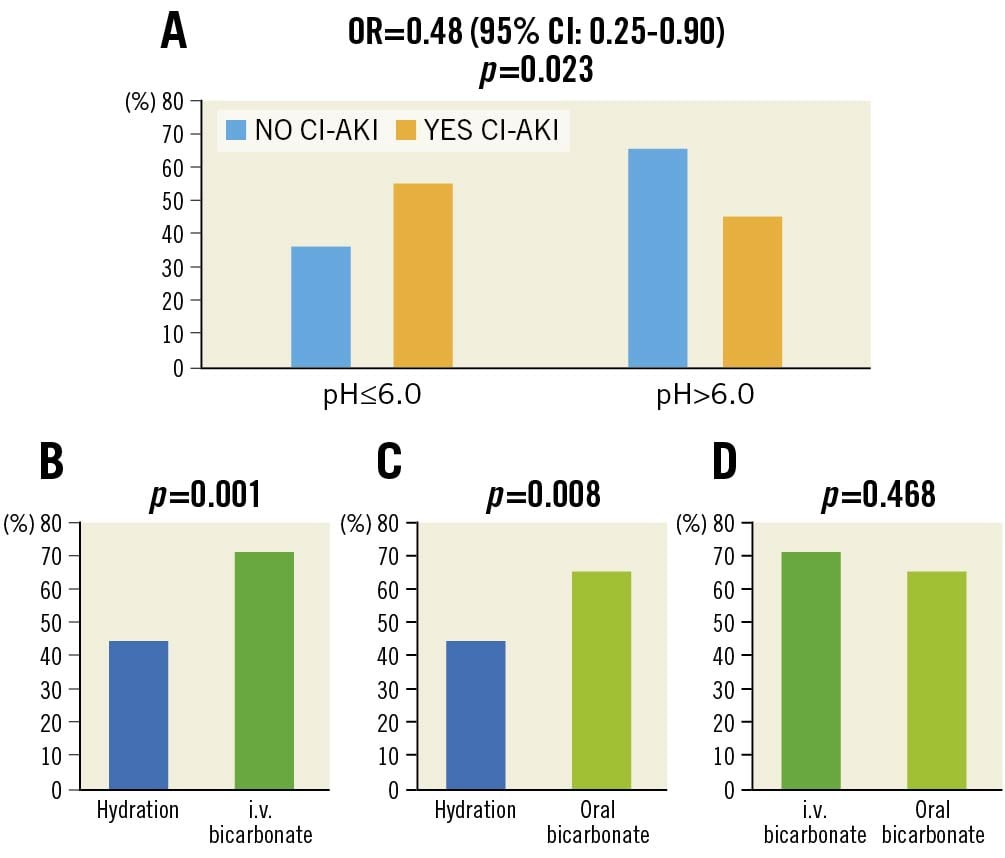
Figure 2. Incidence of contrast-induced acute kidney injury (CI-AKI) as a function of urinary pH in the three study groups. The upper panel (A) shows the difference in the incidence of CI-AKI between patients with urinary pH below or above 6. The lower panels (B-D) report the pairwise comparisons of the association of the 3 strategies used in the study with the achievement of urine alkalinisation (pH >6.0). CI: confidence interval; i.v.: intravenous; OR: odds ratio
Secondary endpoints
Patients with a preprocedural urine pH >6.0 were significantly more numerous in the i.v. and oral bicarbonate groups (71% and 65%, respectively) compared with the group receiving hydration alone (44%; p=0.004) (Table 4). Pairwise comparisons between the percentage of patients achieving urinary alkalinisation with i.v. bicarbonate versus hydration, and between oral bicarbonate versus hydration, were both significant (p=0.001 and p=0.008, respectively) (Figure 2). Conversely, differences were not significant between the i.v. and oral bicarbonate groups (p=0.468) (Figure 2).
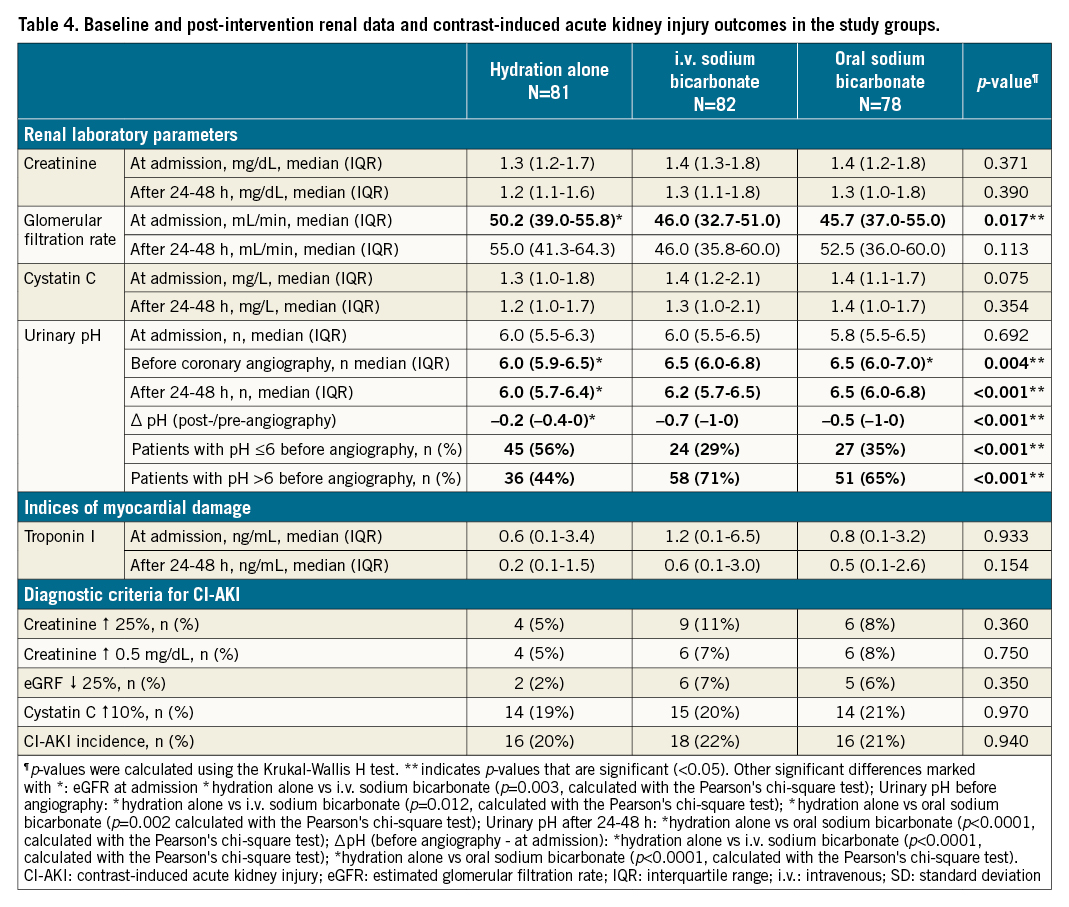
The difference between pre- (at randomisation) and post-angiography urinary pH (Δ pH) was greatest in the i.v. bicarbonate, and significantly different between both bicarbonate groups and the hydration-alone group (p<0.001) (Table 4). A significantly different urinary alkaline status in the 3 study arms was maintained at 24-48 hours after coronary angiography (p<0.001) (Table 4).
The incidence of CI-AKI, however, did not show significant differences in the 3 study groups (20% in the hydration group, 21% in the oral bicarbonate group and 22% in the i.v. bicarbonate group; p=0.94) (Table 4).
Secondary analyses (Supplementary Table 1-Supplementary Table 7) tested bicarbonate efficacy in various patient subgroups that, theoretically, might have benefitted more from bicarbonate treatment in the prevention of CI-AKI, namely:
a. patients with baseline eGFR <60 mL/min (Supplementary Table 1);
b. patients with eGFR <60 mL/min and urinary pH ≤6.0 at entry (Supplementary Table 2);
c. patients with eGFR <60 mL/min, baseline urinary pH ≤6 and preprocedural urinary pH >6 (therefore alkalinised by bicarbonate treatment) (Supplementary Table 3);
d. patients with eGFR <60 mL/min, urinary pH ≤5.5 at entry and preprocedural urinary pH >6 (therefore alkalinised to a greater extent by bicarbonate treatment) (Supplementary Table 4);
e. patients with eGFR ≤45 mL/min and baseline pH <6 (Supplementary Table 5);
f. patients with baseline eGFR ≤60 mL/min and delta pH (preprocedural - baseline pH) ≥0.5 (Supplementary Table 6);
g. patients with baseline eGFR ≤45 mL/min and delta pH (preprocedural - baseline pH) ≥0.5 (Supplementary Table 7).
These subanalyses were all intended to be only hypothesis-generating due to the small number of patients in the subclasses explored. None of these could uncover hints as to the specific groups sensitive to bicarbonate administration in preventing the development of CI-AKI according to treatment allocation. This was the case both considering the bicarbonate groups together vs the control group, as well as (not shown) the two bicarbonate groups separately.
In models developed to predict the occurrence of CI-AKI considering preprocedural urine pH, eGFR at entry and either oral or i.v. bicarbonate; or preprocedural urine pH, eGFR at entry and oral and i.v. bicarbonate combined, bicarbonate administration could never significantly predict the lesser occurrence of CI-AKI (Supplementary Table 8, Supplementary Table 9, respectively).
Despite some discrepancies between GFR estimates with the MDRD formula or cystatin C measurement, there were no differences in cystatin C values in the groups compared, consistent with findings in the main study (Table 4).
Safety
No adverse events other than CI-AKI (and, specifically, no fluid congestion-related adverse events, such as pulmonary oedema or other symptoms or signs of acute heart failure) occurred in the 3 study groups throughout the observation time. We did not engage in longer follow-ups.
Discussion
In this study, involving patients with moderate and severe CKD who underwent coronary angiography with/without PCI, we found that preprocedural urine alkaline status, defined as featuring a urinary pH >6, was associated with a less frequent development of CI-AKI than in the non-alkaline status. We also showed that both i.v. bicarbonate and oral bicarbonate were more effective than hydration alone in achieving urine alkalinisation, with no major differences between the two modalities of bicarbonate administration. We found, however, no statistically significant differences in CI-AKI in the 3 study arms.
Although based on quite uncertain data in literature, bicarbonate is still currently widely used in clinical practice to prevent CI-AKI10. Most studies reporting a favourable effect of bicarbonate are, however, underpowered, and the claim of efficacy may have been subject to a positive-result bias11. In contrast to most trials, the Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial11 involved approximately 5,000 individuals receiving a radiologic contrast medium predominantly for diagnostic reasons. In this trial, patients were randomised into 4 groups comparing i.v. bicarbonate with saline and N-acetylcysteine with placebo in a 2-by-2 factorial design. The incidence of CI-AKI was a secondary study endpoint but occurred with comparable frequencies in the 4 groups. Although the study had many advantages over previous published trials, notably the much larger sample size, it also presented limitations. First, the use of a primary endpoint evaluated at 90 days could have masked favourable effects possibly only occurring early in the follow-up. Second, the study did not adhere to a unique protocol for i.v. fluid administration. Third, the study lacked an assessment of preprocedural urinary pH, therefore it did not estimate the degree of urinary alkalinisation, which is the direct effect of bicarbonate and possibly the mediator of nephroprotection. Thus, the unselected administration of bicarbonate to a wide population in whom only a minority of patients would possibly be susceptible to improvement may have diluted a favourable effect.
An overview of the various trials of bicarbonate in the prevention of CI-AKI supports the notion that the achievement of urinary alkalinisation may be involved in the prevention of renal damage: studies measuring urinary pH and showing favourable results were indeed those in which a significant urinary alkaline status was achieved121314, while studies where bicarbonate did not induce adequate urinary alkalinisation were apparently not associated with a better outcome compared with hydration alone. To confirm the hypothesis that the nephroprotective efficacy of bicarbonate could lie in an appropriate preprocedural urinary alkalinisation, Markota et al showed that in patients undergoing coronary angiography, sodium/potassium citrate, another alkalinising agent, significantly but selectively reduced the incidence of CI-AKI compared with hydration alone only in those achieving an adequately high urinary pH8. Such findings were inspiring for the design of the trial here presented.
TEATE overcomes many limitations of previous studies. Preprocedural urinary pH was measured in all patients to assess the alkalinising capacity of the tested strategies; the incidence of CI-AKI was related to the achievement of a precise cut-off of urinary pH; the amount of i.v. fluids administered in the 3 arms was standardised on the basis of patients’ body weight; and variations in infusion rates were strictly planned and controlled according to a detailed protocol. Our study achieved the primary endpoint in demonstrating that the incidence of CI-AKI is significantly lower in patients with an alkaline urine status. This can be interpreted either by postulating that acidic urine is itself a mediator of renal injury; or that factors eventually determining CI-AKI also determine a higher degree of urine acidification.
Our study also achieved one of the two selected secondary endpoints: demonstrating the ability of both oral and i.v. bicarbonate to alkalinise urine, and to a similar degree. Had the third prespecified endpoint been achieved, this finding would have closed the loop of statistical inference also showing the viability of the more practical strategy of oral bicarbonate administration instead of the i.v. infusion for preventing CI-AKI. Our study, however, could not show differences in the incidence of CI-AKI between the control group and the two bicarbonate groups. We undertook several subgroup analyses to understand whether (a) a different definition of the pH threshold to define urine alkalinisation; (b) the pre/post bicarbonate difference in urine pH; or (c) the degree of baseline impairment of renal function – alone or in combination – could uncover specific groups sensitive to bicarbonate administration. Simply, we did not find hints suggesting the viability of any such ancillary hypotheses.
The inevitable conclusion, as depicted in the Central illustration, is that acidic urine is a marker – and not a mediator – of CI-AKI, and is likely to be a side effect of factors determining CI-AKI through their own pathogenetic pathways.
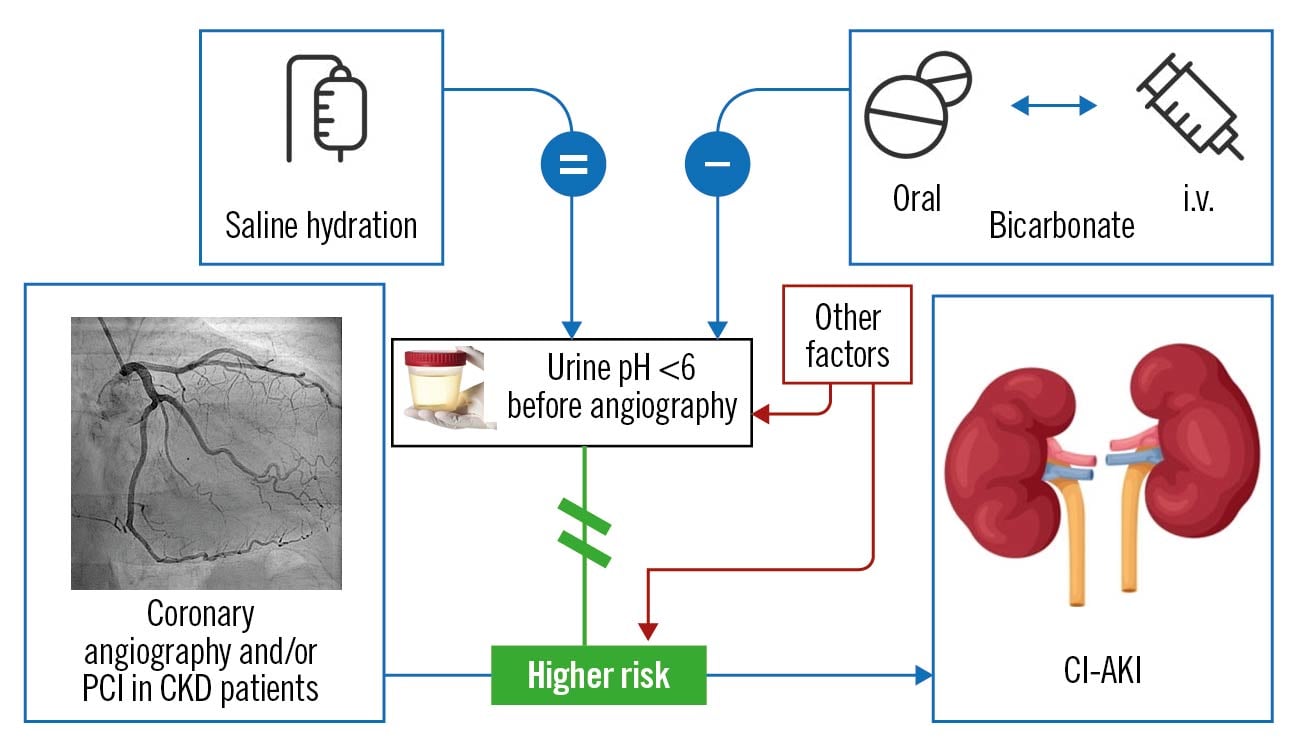
Central illustration. Causal inference on the relationship between bicarbonate administration, urine alkalinisation and the prevention of contrast-induced acute kidney injury (CI-AKI), illustrating the disconnect between urine alkalinisation and prevention of CI-AKI. Bicarbonate – regardless of the administration route, either oral or i.v. – promotes urine alkalinisation (contrasting the occurrence of acidic urine before angiography. The presence of a urine pH >6 (urine “alkaline status”) is associated with a reduced risk of CI-AKI in patients with chronic kidney disease (CKD) undergoing coronary angiography or percutaneous coronary interventions (PCI), but not in a causal relationship, because no change occurred after bicarbonate-induced urine alkalinisation on the risk of CI-AKI, making it necessary to postulate the crucial role of other factors – on the one hand interfering with urine alkalinisation, and on the other hand directly promoting CI-AKI. i.v.: intravenous
The search for such direct mediators of CI-AKI was beyond the purpose of this study, but factors such as anaemia, chronic heart failure/left ventricular dysfunction, age, diabetes, hypotension, volume and type of contrast media are likely to exert renal-damaging effects and, at the same time and apparently independently, favour the occurrence of an acidic urine. As a practical conclusion, this study does not support the preprocedural use of either i.v. or oral bicarbonate to prevent CI-AKI.
Study limitations
We recognise limitations in our study. The sample size was estimated based on previous literature2 to show differences in the rate of CI-AKI as a function of urine alkalinisation, but other estimates on the occurrence of CI-AKI would have suggested a larger sample size. The impracticability of recruitment of a larger study population in a spontaneous study such as ours prompted us not to select the bicarbonate effect on CI-AKI as the primary study endpoint. It is reassuring, however, that we did not see hypothesis-generating trends suggesting the efficacy of bicarbonate in any of the subgroup analyses attempted. We elected to define the occurrence of CI-AKI at day 2, post-procedure, according to a previous report8, and also for organisational reasons. Others have reported a later peak occurrence of CI-AKI7. Another study limitation is the proportion of already alkaline urinary pH at the basal sampling in a large number of study patients: at baseline, indeed, the mean urinary pH of the 3 groups was approximately 6, close to the cut-off value for defining the hypothetical best target population in our study. However, one may counter-argue that no hints toward the efficacy of bicarbonate were found even when restricting subanalyses to patients experiencing a larger change (delta) in urinary pH (Supplementary Table 1-Supplementary Table 9). A fourth limitation is the significantly higher median eGFR value in the hydration control group (50.2 mL/min) vs the i.v. and oral bicarbonate groups (46.0 mL/min and 45.7 mL/min, respectively), indicating a population at slightly lower risk of CI-AKI in the control group. Although statistically significant and most likely due to the play of chance, this minimal difference, not reflected in differences in serum creatinine or cystatin C, is felt to be unlikely to explain the neutral results of bicarbonate administration on the occurrence of CI-AKI. A fifth limitation is the definition of CI-AKI itself which was in use at the time of the study conception, but was later superseded by the Kidney Disease Improving Global Outcomes (KDIGO) definition, currently in use10. We feel that this change in definition does not detract from the main study conclusions.
Conclusions
Urinary pH before the administration of contrast medium appears to be an inverse correlate of CI-AKI incidence; and bicarbonate is superior to hydration alone in achieving urinary alkalinisation. Since, however, bicarbonate did not reduce the incidence of CI-AKI, urinary pH appears to be a marker and not a mediator of CI-AKI.
Impact on daily practice
Urinary pH before the administration of contrast medium appears to be an inverse correlate of CI-AKI incidence. Sodium bicarbonate, either oral or i.v., is superior to hydration alone in achieving urinary alkalinisation, but not in preventing CI-AKI. Urinary pH is likely to be a marker, and not a mediator, of CI-AKI.
Acknowledgements
We would like to thank the medical personnel and the nurses in the 3 hospitals where this investigation was carried out for supporting this investigator-initiated study. We dedicate this study to Dr Luciano Moretti†, Chief of Cardiology at the participating Mazzoni Hospital, who died during the conduct of this study. TEATE, the acronym of this study, is the ancient name of the city of Chieti, Italy.
Conflict of interest statement
R. De Caterina received grants or contracts from Daiichi Sankyo, Novartis, and Roche (outside of and unrelated to the present manuscript); consulting fees from Boehringer Ingelheim, Bayer, BMS/Pfizer, Janssen, Daiichi Sankyo, Novartis, AstraZeneca, Milestone, Lilly, Roche, Menarini, and Guidotti; honoraria from Boehringer Ingelheim, Bayer, BMS/Pfizer, Janssen, Daiichi Sankyo, Novartis, AstraZeneca, Milestone, Lilly, Roche, Menarini, and Guidotti; support for attending meetings and/or travel from Daiichi Sankyo and Menarini; and is on the European Society of Cardiology (Nominating Committee). U. Limbruno received consulting fees from UVET-GBT; honoraria from Eureka, Summeet, and Clinical Forum; and is on the GISE National Board (2015-2021). G. Renda received honoraria from Bayer, Boehringer Ingelheim, AstraZeneca, BMS-Pfizer, and Daiichi Sankyo; support for attending meetings and/or travel from Bayer, BMS-Pfizer, Boehringer Ingelheim and Daiichi Sankyo. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

