Abstract
Aims: Contrast-induced nephropathy (CIN) is a leading cause of morbidity and mortality in patients undergoing percutaneous coronary intervention (PCI). Limited data, however, are available on predictors of CIN in PCI for chronic total occlusion (CTO) lesions. The aim of the study was to determine the risk of developing CIN in patients undergoing CTO PCI by studying the effects of clinical variables, interventional techniques, and CTO lesion characteristics on renal function.
Methods and results: This retrospective analysis included consecutive patients referred for CTO PCI between January 2002 and December 2009. CIN was defined as an elevated serum creatinine level ≥25% of baseline serum creatinine level at 48-72 hours after procedure. Patient characteristics, Mehran score, lesion characteristics, interventional procedure, and devices used were compared between CIN and non-CIN groups. For the 516 patients eligible for analysis, the incidence of CIN was 5.4% (28/516). Two patients needed transient haemodialysis (0.4%, 2/516). Analysis of risk using Mehran scoring found that the incidence of CIN was 0.5% (1/207) among low-risk patients, 3.4% (7/205) among moderate-risk patients, 15.9% (14/88) among high-risk patients and 37.5% (6/16) among very high-risk patients. The Mehran score high-risk group (11-15) and the very high-risk group (≥16) were definitely predictors of CIN after CTO PCI (OR: 27.022 [95% CI: 2.787-262.028, p=0.004]; OR: 32.512 [95% CI: 2.149-491.978, p=0.012]). Severe tortuosity was the only predictor of CIN after CTO PCI in angiographic and procedural findings (OR: 6.621 [95% CI: 1.090-40.227, p=0.040]).
Conclusions: Being in the Mehran score high-risk group (11-15) or the very high-risk group (≥16) and severe tortuosity were predictors of CIN after CTO PCI.
Introduction
The incidence of contrast-induced nephropathy (CIN) in the general population is estimated to be between 1% and 6%1, and is even higher when CIN follows percutaneous coronary intervention (PCI). According to a retrospective analysis performed by the Mayo Clinic for the general population undergoing PCI, the incidence of CIN was 3.3% and the need for dialysis was 0.3%2. However, the incidence of CIN was higher in patients with pre-existing impaired renal function3, and was >50% in very high-risk patients4. Furthermore, CIN accounts for 11% of acute renal failure (ARF) cases and is the third leading cause of hospital-acquired ARF, contributing to prolonged hospital stay5 and increased medical costs6,7.
The in-hospital mortality rate was 22% for patients who developed CIN, compared with only 1.4% of in-patients who did not develop CIN8. Therefore, it is important to identify patients at high risk for developing CIN prior to PCI. Although pre-existing renal dysfunction is the most important risk factor, many other risk factors are associated with CIN9-11. Clinical variables such as age, female gender, diabetes mellitus (DM), presence of hypotension, anaemia, congestive heart failure, and low left ventricular ejection fraction (LVEF) were found to play a role. In addition, procedural variables such as intra-aortic balloon pump (IABP) use, volume of contrast agents used, and urgent need for PCI were also important factors12-14. Based on these findings, Mehran and her colleagues assigned a scoring system which integrated eight variables to predict CIN after PCI4.
The technical success rate of PCI for coronary chronic total occlusion (CTO) lesions has steadily increased over the past 15 years because of improved operator expertise, and improvements in both equipment and procedural techniques15. Complex CTO lesions hampered initial success rates with prolonged x-ray exposure and use of large volumes of contrast medium16, leading to CIN in some cases. Although several studies on the incidence of CIN in CTO intervention have been reported16,17, limited data are available on the predictors of CIN in PCI performed for CTO lesions16.
We assessed the clinical and procedural variables, as well as the Mehran score, in a large number of patients who underwent PCI in order to determine the predictors of CIN in CTO PCI.
Materials and methods
PATIENT ENROLMENT AND EXCLUSION CRITERIA
This retrospective study protocol was approved by the institutional review board of Kaohsiung Chang Gung Memorial Hospital, Taiwan. All patients gave their written informed consent prior to participation in the study.
This is a single-centre, single-operator registry comprising patients with documented CTO lesions under planned revascularisation. CTO was defined as Thrombolysis In Myocardial Infarction (TIMI) grade 0 for more than three months, with the presence of typical angina or reversible myocardial ischaemia on thallium stress study. Exclusion criteria were: 1) end-stage renal failure; 2) recent myocardial infarction or unstable haemodynamics; 3) total occlusion of bypass grafts.
During the period from January 2002 to December 2009, 516 consecutive patients who underwent PCI for CTO of a native coronary artery were included. The baseline demographics, angiographic characteristics, procedural outcomes, and CIN were examined in all 516 patients. All procedural coronary angiograms were reviewed to assess the anatomy and morphology of CTO segments and grade of collaterals.
DEFINITIONS
The duration of occlusion was estimated by a history of angina, history of MI in the same territory, or previous angiography. The characteristics of each CTO lesion were defined according to the SYNTAX scoring system based on the complexity of coronary artery disease18. Severe calcification of a CTO lesion was defined if multiple persisting opacifications of the coronary wall were visible in more than one projection surrounding the complete lumen of the coronary artery at the site of the lesion. Severe tortuosity was defined if there was one or more bend of 90° or more, or three or more bends of 45° to 90° proximal to the diseased segment. Good collateral circulation was defined as TIMI 3 collateral flow. The length of the CTO was measured following bilateral simultaneous coronary injections in cases with collaterals from other coronary arteries, or antegrade coronary injection in cases with bridging collaterals, visualising the filling of both the proximal and the distal occluded artery.
Angiographic success was defined as residual stenosis ≤30% by visual analysis in the presence of TIMI grade 3 flow. In addition, the distal wire position was documented as being in the true lumen either by a coronary angiogram or by intravascular ultrasound (IVUS) examination. The retrograde approach was defined as the introduction of the guidewire into the collateral channels (CCs), which connected to the target CTO vessel distal to the lesion.
Each patient was pretreated with aspirin and clopidogrel and administered weight-adjusted heparin to keep the activated clotting time (ACT) more than 250 seconds. During the interventional procedure, either iohexol, a non-ionic, iodinated, low-osmolar radiologic contrast agent, or iodixanol (Visipaque, 320 mg iodine/ml; Amersham Health, Princeton, NJ, USA), a non-ionic, iso-osmolar (290 mOsm/kg water) contrast agent, was used.
The eGFR was calculated based on the preprocedural serum creatinine level in mg/dL, age in years, weight in kilograms, and gender, using the Cockcroft-Gault formula as follows19:
eGFR (ml/min/1.73 m2)=([140-age]×weight)/(serum creatinine×72) (×0.85, if the patient is female).
Serum creatinine (SCr) was measured at 24 hours before the procedure and between 48 and 72 hours after the procedure. Additional SCr measurements were obtained in patients with initial elevation of SCr or post-procedural deterioration of baseline renal function or in patients with prolonged hospitalisation for other reasons (haemorrhage, ischaemia, etc.). CIN was defined as an increase in the baseline SCr levels ≥25% in the 48-72 hours following the index procedure. The predictive risk score of CIN was assessed on the basis of the patient`s clinical and laboratory findings, as previously proposed4. Patients were stratified into four groups of increasing risk for CIN according to a validated risk scoring system (Mehran score)4, which includes variables of age, gender, eGFR (or SCr), haematocrit, diabetes mellitus, presence of congestive heart failure, contrast volume, hypotension, and support of intra-aortic balloon pump (IABP). The Mehran scoring groups included low risk (≤5 points), moderate risk (6-10 points), high risk (11-15 points), and very high risk (≥16 points).
The guidewires used included Miracle 3g (ASAHI Intecc, Aichi, Japan), Miracle 6g (ASAHI Intecc), Miracle 9g (ASAHI Intecc), Conquest (ASAHI Intecc) and Conquest Pro (ASAHI Intecc), called stiff wires. Microcatheters included Transit catheters (Cordis, Johnson & Johnson, Warren, NJ, USA), FineCross™ catheters (Terumo Corp., Tokyo, Japan), and Ryujin™ over-the-wire balloon catheters (Terumo Corp.).
STATISTICAL ANALYSIS
Baseline patient characteristics, angiographic data and parameters during each procedure were compared between patients who experienced CIN vs. non-CIN patients. Variables were reported as mean±SD for continuous variables or as percentages for categorical variables. The chi-square test was used for categorical variables, and Student’s t-test was used for continuous variables. Predictors of developing CIN were independently analysed according to three groups of clinical, procedural, and angiographic variables. Stepwise univariate logistic regression analysis was also performed for the possible significant predictors. We included parameters that were found with a significant difference in univariate logistic analysis into our multivariable analysis. A p-value <0.05 was considered statistically significant for all calculations. Statistics were performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
Results
POPULATION DEMOGRAPHICS
A total of 516 patients (mean age: 62.9±11.28 years) were enrolled in this study. The majority of patients were male (85.5%) and 28 (5.4%) patients developed CIN after the index procedure. Their baseline clinical characteristics are detailed in Table 1.
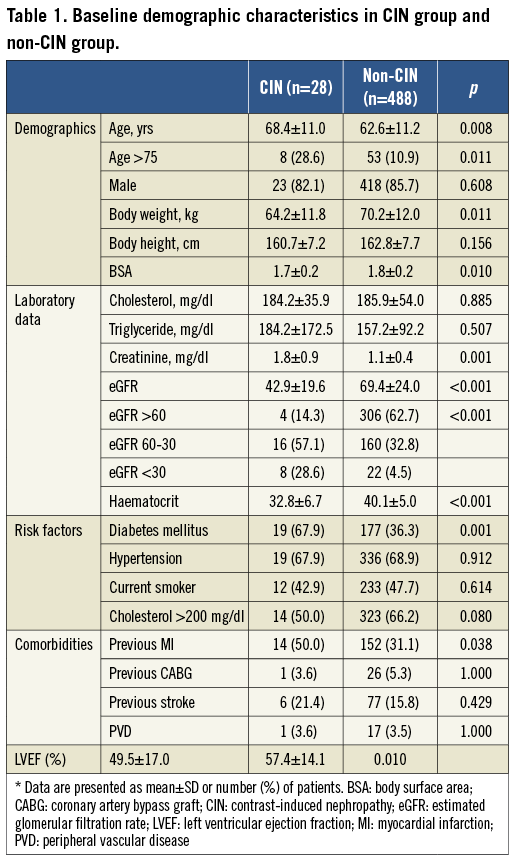
When comparing CIN patients vs. non-CIN patients, we found that those patients who developed CIN were older (68.4±11.0 vs. 62.6±11.2; p=0.008), had lower body weight (64.2±11.8 vs. 70.2±12.0 kg; p=0.011), and lower body surface area (1.7±0.2 vs. 1.8±0.2 m2, p=0.01). In addition, there were more patients with DM (67.9% vs. 36.3%; p=0.001) and previous myocardial infarctions (50.0% vs. 31.1%; p=0.038) in the CIN group. Furthermore, blood biochemistry showed that the CIN group presented with higher serum creatinine levels (1.8±0.9 vs. 1.1±0.4; p=0.001), lower eGFR levels (42.9±19.6 vs. 69.4±24.0, p<0.001), lower haematocrit levels (32.8±6.7 vs. 40.1±5.0; p<0.001) and lower left ventricular ejection fractions (LVEFs) (49.5±17.0 vs. 57.4±14.1; p=0.01).
LESION AND PROCEDURAL CHARACTERISTICS
The characteristics of coronary artery and CTO lesions are shown in Table 2. The majority of patients had multivessel disease which was balanced in both groups (85.8% in the CIN group vs. 74.6% in the non-CIN group). The incidence of CTO target lesions was predominantly in the left anterior descending (LAD) and right coronary artery (RCA) in this study without significant differences between the groups (p=0.258). The CTO lesions were commonly de novo in both groups (85.7% in the CIN group vs. 82.6% in the non-CIN group, p=0.902). The major difference between groups was the severe tortuosity of the CTO vessel (CIN group vs. non-CIN group, 92.9% vs. 75.2%, p=0.033). CTO lesions with blunted occlusion were more prevalent in the non-CIN group compared to the CIN group (45.7% vs. 28.6%, p=0.076).
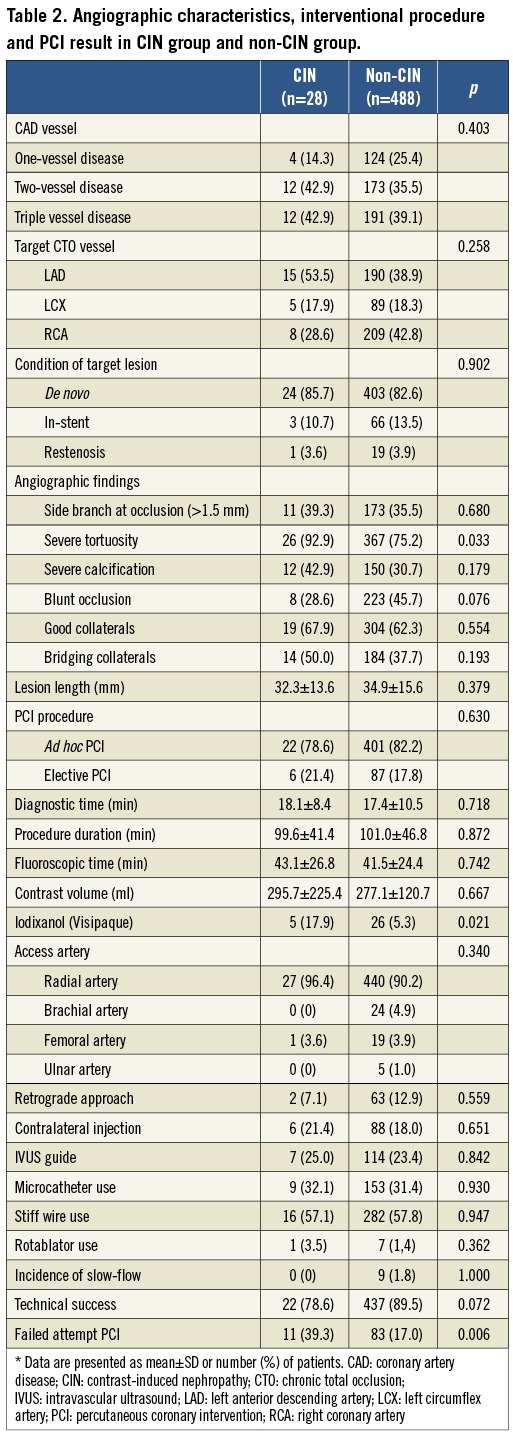
All interventional procedural characteristics are also listed in Table 2. There was no difference in ad hoc or elective procedures between groups (78.6% in the CIN group vs. 82.2% in the non-CIN group, p=0.630). There was no significant difference in fluoroscopic time, procedure duration or contrast volume between the two groups, except for the type of contrast medium used (iodixanol was more frequently used in the CIN group compared to the non-CIN group (17.9% vs. 5.3%, p=0.021). The predominant access route was the transradial approach (>90%), but no difference was found between groups. There was also no difference in the approach manner used (retrograde approach, contralateral injection or IVUS-guided approach) or new devices used (microcatheter and stiff wire) between groups. In addition, the frequency of rotablator use or the incidence of slow-flow was similar in both groups.
Our technical success rate was relatively lower in the CIN group (78.6% vs. 89.5%, p=0.072) without statistical difference. However, the incidence of failed attempt PCI was significantly higher in the CIN group (39.3% vs. 17.0%, p=0.006).
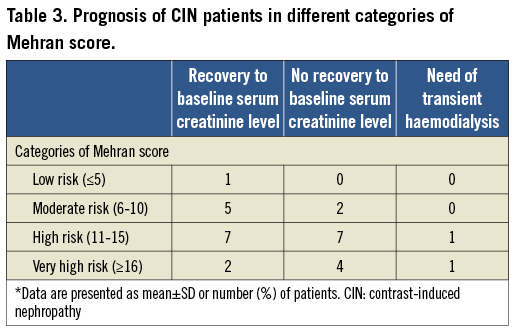
The entire population was separated into four groups according to risk using the Mehran scoring system (Figure 1). For the different Mehran risk categories, the incidence of CIN was 0.5% (1/207) among the low-risk category, 3.4% (7/205) among the moderate-risk category, 15.9% (14/88) among the high-risk category, and 37.5% (6/16) among the very high-risk category. However, two CIN patients required transient haemodialysis. One of these patients was in the high-risk category (1.1%, 1/88) and the other was in the very high-risk category (6.3%, 1/16). The serum creatinine levels obtained 1~3 months following the intervention showed that most of the patients in low and moderate-risk categories regained renal function while the majority of the patients in high and very high-risk categories did not (Table 3).
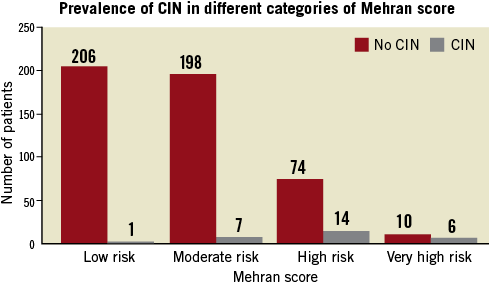
Figure 1. Prevalence of contrast-induced nephropathy (CIN) in different categories of Mehran score.
PREDICTOR EVALUATION
Predictors of developing CIN were independently analysed according to three groups of clinical, procedural, and angiographic variables. Stepwise univariate logistic regression analysis was also performed. The possible significant predictors are summarised in Table 4. There were no significant differences between groups in gender, body height, hypertension, current smoking, dyslipidaemia, history of bypass graft, history of stroke, history of peripheral vascular disease and evidence of heart failure. Also, there were no significant differences in side branch lesion, blunt stump lesion, severely calcified lesion, and CTO lesion length, with good collaterals, with the retrograde approach, with intravascular ultrasound guidance or with rotablator use.
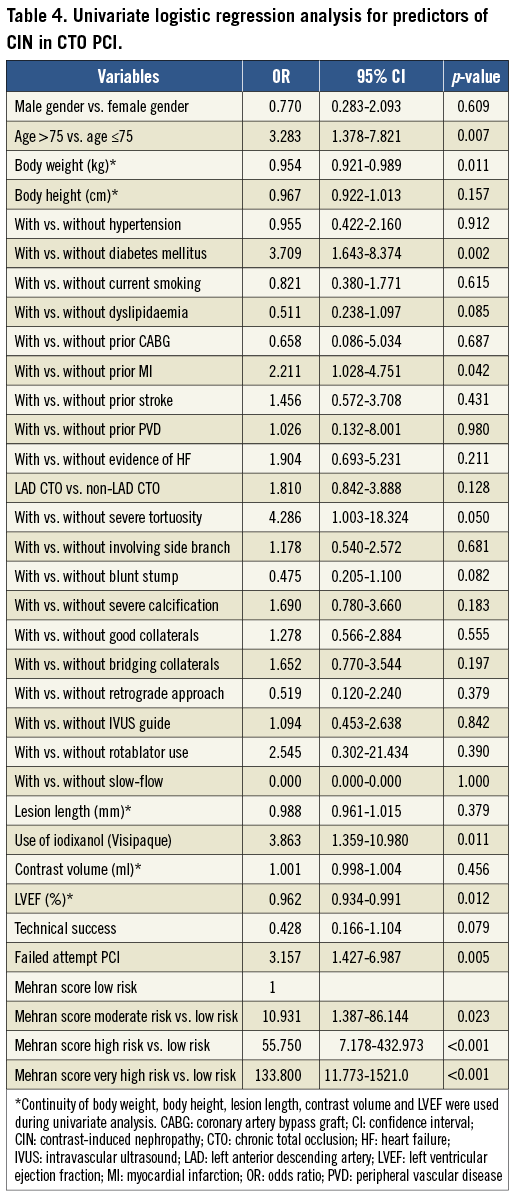
Since the low-risk Mehran score category represented the baseline risk for developing CIN, we found a significant difference in the risk for developing CIN in the moderate-risk Mehran score category (OR: 10.931; [95% CI: 1.387-86.144, p=0.023]). Similarly, an approximately 55.750-fold increased risk (95% CI: 7.178-432.973, p<0.001) for developing CIN was found in the high-risk category and an approximately 133.800-fold increased risk (95% CI: 11.773-1,521.0, p<0.001) for developing CIN was found in the very high-risk category, when compared to the low-risk category in univariate logistic analysis. Regarding baseline and clinical variables, severe tortuosity was the only predictor in angiographic findings for the development of CIN (OR: 4.286; 95% CI: 1.003-18.324, p=0.05) in univariate logistic analysis. The use of iodixanol (Visipaque) contrast and failed attempt PCI were other predictors for the development of CIN (OR: 3.863; 95% CI: 1.359-10.980, p=0.011, and OR: 3.157; 95% CI: 1.427-6.987, p=0.005, respectively).
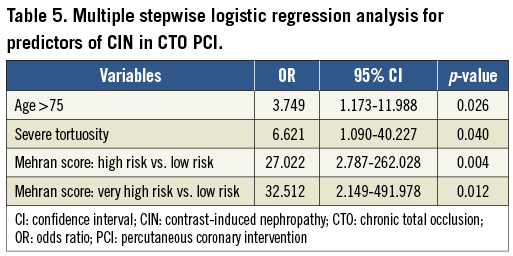
Parameters that were found significantly different in univariate logistic analysis were included in our multivariable analysis (Table 5). The Mehran score high-risk group (11-15) and very high-risk group (≥16) were definitely predictors of CIN after CTO PCI (OR: 27.022 [95% CI: 2.787-262.028, p=0.004]; OR: 32.512 [95% CI: 2.149-491.978, p=0.012]). Severe tortuosity was the only predictor of CIN after CTO PCI in angiographic and procedural findings (OR: 6.621 [95% CI: 1.090-40.227, p=0.040]).
Discussion
CIN is an important issue in the development of in-hospital ARF and it is a well-known complication and leading cause of mortality after coronary intervention. According to the literature, the incidence of CIN is approximately 13%4 in coronary intervention but few studies have focused on the prevalence of CIN in CTO intervention.
PCI for CTO is one of the major challenges for interventional cardiologists due to the complexity of lesions and the high failure rate20. Procedural success is primarily dependent on the operator’s technique and experience21. Therefore, some studies have concluded that CTO intervention requires “super-specialists” who are able to concentrate their time and experience in this one area22. For this reason, we recruited a highly experienced interventional cardiologist who performs high volumes of this particular procedure.
In addition, we analysed nearly all parameters which might affect CIN, including clinical, procedural, and CTO characteristics. From the univariate analysis of baseline characteristics and laboratory findings, significant differences between non-CIN and CIN groups were assumed to affect the development of CIN. For this reason, we assessed an integer of many variables in predicting development of CIN, and so the Mehran score was chosen.
Aguiar-Souto et al described the incidence and predictors for CIN in CTO PCI16. They concluded that CTO intervention is a procedure associated with CIN in 6.16% of patients and is associated with CIN in fewer than 7.0% of patients in moderate or high-risk categories based on Mehran scoring. Our study showed a similar prevalence of CIN (5.4%) after CTO PCI to that found in Aguiar-Souto’s report16 but, compared to their report, our study involved twice the volume of patients and all procedures were performed by a single, well experienced interventionalist. The reason for the low incidence of CIN reported by Aguiar-Souto et al may be due to the higher proportion of patients (79.8%) estimated to be low (40.1%) or moderate (39.7%) risk for developing CIN and also adequate hydration in their high-risk patients. According to the literature, adequate intravenous hydration can reduce the incidence of CIN in patients with renal dysfunction23,24. In addition, although the protective effect of iodixanol contrast in high-risk patients remains controversial, a meta-analysis25 showed that the use of non-ionic low-osmolar contrast media has been associated with fewer renal adverse effects than high-osmolar contrast media in patients with previous chronic kidney disease. Furthermore, a randomised trial26 suggested the benefits of non-ionic iso-osmolar contrast (iodixanol) over low-osmolar contrast (iohexol). On the other hand, procedural duration also increases the risk for CIN, but the ratio of contrast volume/procedure duration (ml/min) was not an independent predictor among patients with chronic kidney disease27.
The Aguiar-Souto study16 showed that clinical parameters, procedural characteristics, target vessels, and even risk categories for Mehran scoring were not predictors for CIN in CTO intervention. In our study, on the contrary, many of the parameters studied showed significant differences between non-CIN vs. CIN groups. These CIN predictors were similar to those found in coronary artery lesion interventions in general4,12. Furthermore, using multivariable analysis, our study showed that the Mehran scoring system was also an independent predictor for developing CIN during CTO PCI in high-risk and very high-risk categories. In addition, the majority of CIN patients (53.6%; 15/28) recovered their baseline renal function within three months, even those who were in the high-risk categories (50%; 7/14). Therefore, we can conclude that the risk of developing CIN in CTO PCI is relatively low and the Mehran scoring system is a good predictor for CIN in CTO PCI.
After multivariable analysis of procedural variables, the type of contrast used was the only parameter found to affect CIN, but this finding differed greatly from what has been reported in the literature. Iodixanol showed a significant risk for CIN in this study, while it is no worse than iohexol in preventing CIN as has been shown in previous studies26,28,29. However, after adjustment for the multivariable analysis of all parameters, including clinical characteristics, the effect of contrast agent was found to be due to bias and therefore eliminated, since iodixanol was only used in patients with renal dysfunction combined with or without diabetes.
When evaluating parameters using multivariable analysis, severe tortuosity remained a significant risk factor with a relative risk that was 6.621 times greater compared to cases without severe tortuosity (p=0.040; OR: 6.621; 95% CI: 1.090-40.227). After dividing patients into groups based on the presence or absence of severe tortuosity, we found that the severe tortuosity group had a higher percentage of patients with renal dysfunction (as defined by a creatinine cut-off value >1.5 [15.3% vs. 7.3%; p=0.024]), calcification of coronary arteries (34.6% vs. 21.1%; p=0.005) and greater lesion length (mean: 35.6 mm vs. 32.3 mm; p=0.027). In addition, although the volume of contrast used was larger in the tortuosity group compared to the non-tortuosity group (mean: 282.2 ml vs. 263.8 ml; p=0.181), it did not achieve statistical significance. All of the evidence pointed to severe tortuosity as an independent risk factor for CIN.
The major reason for CTO PCI failure was the inability of the guidewire to cross the CTO lesion20. Several interventional devices have recently been developed to solve this problem including stiff wire and microcatheters. The stiff wire was developed to penetrate the calcified fibrous cap of CTOs30, and microcatheters were developed to provide additional support during guidewire passage21. These two devices have contributed to the increased success rates of CTO PCI22. Thus, we hypothesised that these devices would be helpful in preventing CIN. Unfortunately, the effect of the device in preventing CIN did not appear to be significant in this study. Further analysis of cases using microcatheters vs. stiff wire showed that microcatheters were used more in cases with severe tortuosity (p=0.032) and longer lesion length (38.0 mm vs. 33.3 mm, p=0.004). A stiff wire was used to a greater extent in cases with calcification (p=0.004), blunted lesions (p<0.001), severe tortuosity (p=0.001) and longer lesion length (37.6 mm vs. 31.0 mm; p<0.001). In this study, only severe tortuosity was a significant predictor of CIN. Thus, the effect of stiff wire and microcatheters in preventing patients with CTO lesions from developing CIN and their effect on risk of CIN were eliminated.
Limitations
Our study had several limitations. Firstly, it is a retrospective design study which increases the risk of bias. Secondly, SCr was evaluated between 48 hours and 72 hours. Some studies have reported that SCr levels may peak at three days after contrast medium administration and return to normal within 10 days31. Therefore, this study may have missed the peak Cr levels in some patients. However, this factor would involve only a limited number of patients who developed CIN because Cr levels usually increase 24 hours after contrast administration32. Also, compared to baseline, the absolute (≥0.5 mg/dL) and relative increases (≥25%) in SCr, at 48-72 hours after exposure to a contrast agent, were known to predict clinical outcomes27 and thus define CIN, clinically. We chose the relative increase in SCr to define CIN in this study, similar to the definition used by Mehran et al for their scoring system. However, one study showed that an SCr of ≥0.5 mg/dL was more sensitive to long-term mortality and morbidity in coronary intervention33. Further studies are needed to resolve this issue. Thirdly, only one highly experienced CTO interventional cardiologist participated in this study. Although the use of one interventionist could potentially eliminate the bias created by multiple operators, this also raises the question as to whether the predictors of CIN determined by our study can be generalised to all coronary interventionists.
Conclusions
The Mehran score high-risk group (11-15) and the Mehran score very high-risk group (≥16) were definite predictors of CIN after CTO PCI. Severe tortuosity was the only predictor of CIN after CTO PCI in angiographic and procedural findings.
| Impact on daily practice Contrast-induced nephropathy (CIN) is a leading cause of morbidity and mortality in patients undergoing percutaneous coronary intervention (PCI). However, limited data are available on predictors of CIN in PCI for chronic total occlusion (CTO) lesions. In our conclusions, the Mehran score high-risk group (11-15), Mehran score very high-risk group (≥16) and angiographic severe tortuosity were the predictors of CIN in CTO PCI. In our daily practice, more careful hydration before and after the procedure, lower contrast volumes during the procedure and precise follow-up after the procedure should be applied to these high-risk patients. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

