
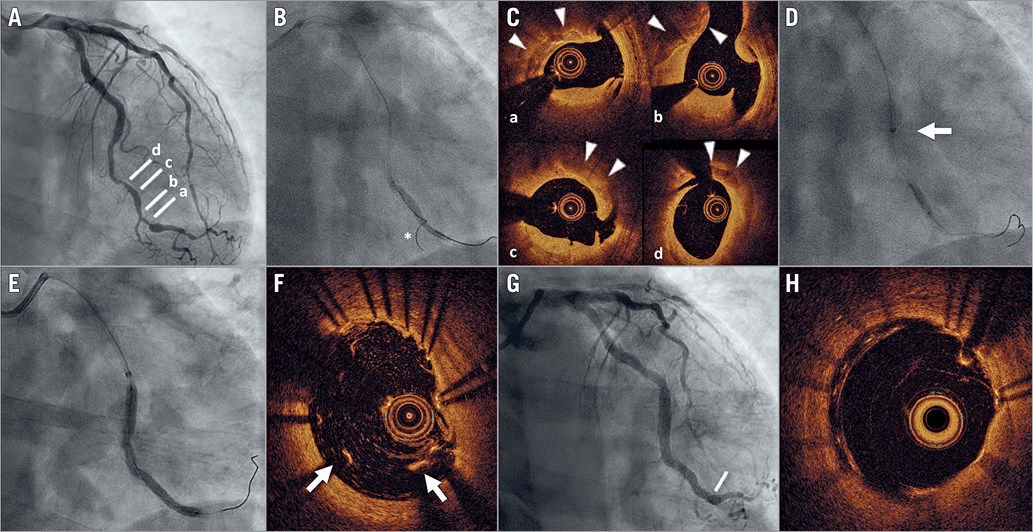
Figure 1. Ultra-low contrast PCI. A) Coronary angiography demonstrates a long and tortuous lesion in the distal circumflex. B) Predilatation with 2.5×30 mm semi-compliant balloon. The asterisk indicates the wire advanced into a small side branch and used to create a “roadmap” for PCI. C) OCT shows extensive calcification (arrowheads) and multiple dissection planes (cross-sections at the levels indicated in panel A). D) Atherectomy with 2.5 mm scoring balloon (arrow indicates mother-and-child catheter). E) Implantation of 3.5-3.0×50 mm sirolimus-eluting stent. F) OCT imaging shows major malapposition (arrows) of the stent at the distal edge (corresponding to the level indicated in panel G). Good final angiographic (G) and OCT (H) result after repeat high-pressure post-dilatation.
A 52-year-old man with a prior kidney transplant and chronic kidney disease (CKD) (baseline creatinine 2.7 mg/dl; estimated glomerular filtration rate [eGFR] 25 ml/min/1.73 m2) presented with angina. Coronary angiography demonstrated two-vessel disease, with a long and tortuous lesion in the distal circumflex and diffuse disease in the distal left anterior descending, which was not considered to be amenable to treatment (Figure 1A, Moving image 1). The patient was referred for percutaneous coronary intervention (PCI) on the circumflex.
Our ultra-low contrast (ULC) PCI protocol is used in patients with eGFR <30 ml/min/1.73 m2 and comprises the use of: 1) ioversol, a low-osmolar contrast medium, diluted at 50%, and used only in key moments of the procedure (e.g., to confirm the final result); 2) side branch wiring to create a “roadmap” for PCI; 3) optical coherence tomography (OCT) with dextran 40 (instead of contrast) to guide PCI (flow rate: 4.0 ml/s, total volume: 14.0 ml, pressure: 400 psi, rise time: 0.0 s). Additionally, patients receive hydration with isotonic saline (1.5 ml/kg/hr for 12 hours before and 24 hours after PCI).
A 6 Fr XB 3.5 guiding catheter was inserted transradially to engage the left main. Two wires were placed in the distal circumflex and one of its branches. After a small balloon was advanced with difficulty (due to marked tortuosity) and predilatation was performed (Figure 1B), a mother-and-child catheter was advanced into the distal circumflex. Dextran-based OCT was performed and revealed extensive calcification (Figure 1C, Moving image 2). Scoring balloon angioplasty was performed (Figure 1D), and a 3.5-3.0×50 mm sirolimus-eluting stent (BioMime Morph™; Meril Life Sciences Pvt., Vapi, India) was implanted (Figure 1E) and post-dilated. Repeat OCT showed major stent malapposition at the distal edge (Figure 1F), which was resolved with repeat high-pressure post-dilatation. Final angiographic (Figure 1G, Moving image 3) and OCT (Figure 1H, Moving image 4) results were good. Contrast volume was 12.5 ml, fluoroscopy time 35 min, and radiation dose 65 Gy·cm2. Creatinine at 48 and 72 hours remained at 2.7 mg/dl. At 30-day follow-up, the patient was asymptomatic and had not suffered any hospitalisation.
The incidence of contrast-induced nephropathy (CIN) in patients with severe CKD is high (26.6%), and its consequences devastating, including a high risk of in-hospital mortality (9.7%)1. CKD subjects undergo PCI less frequently than non-CKD patients, due to aversion to the risk of CIN. In such a setting, the implementation of ULC-PCI protocols is warranted. Diluted contrast is a feasible option to reduce contrast volume in PCI while maintaining sufficient image quality2. Dextran has been shown to be effective and safe for blood flushing during OCT3. Zero-contrast4 and low-contrast2 PCI protocols based on intravascular ultrasound (IVUS) have the potential to decrease the incidence of CIN, although IVUS significantly prolongs these procedures and has lower resolution compared with OCT. Additionally, a zero-contrast approach might prevent the prompt diagnosis of life-threatening complications, such as distal-vessel perforation. Therefore, ULC-PCI based on diluted contrast and dextran-based OCT appears to be an appealing option in patients with advanced CKD and deserves further investigation.
Conflict of interest statement
L. Azzalini reports grants from ACIST Medical Systems, and personal fees from Guerbet, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Baseline coronary angiogram (non-diluted contrast).
Moving image 2. Dextran-based optical coherence tomography after predilatation.
Moving image 3. Final angiographic result (diluted contrast).
Moving image 4. Final dextran-based optical coherence tomography.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Baseline coronary angiogram (non-diluted contrast).
Moving image 2. Dextran-based optical coherence tomography after predilatation.
Moving image 3. Final angiographic result (diluted contrast).
Moving image 4. Final dextran-based optical coherence tomography.

