
Abstract
Aims: A systematic review and a meta-analysis were performed to define better the role of statin use prior to angiography in preventing contrast-induced acute kidney injury (CI-AKI).
Methods and results: MEDLINE, Embase, Cochrane Library, references from review articles, and conference proceedings were searched, with no language restriction, for randomised controlled trials (RCT) evaluating the use of statin therapy prior to angiography for the prevention of CI-AKI. Nineteen RCTs including 7,161 patients were identified. The pooled analysis demonstrated a significant reduction in the incidence of CI-AKI in patients treated with statin prior to invasive angiography when compared with control (RR 0.52; 95% CI: 0.40-0.67). Patients with chronic kidney disease stage 3 or worse were largely underrepresented in these trials, and statin therapy did not significantly reduce the risk of CI-AKI in the three studies which enrolled a patient population with a mean eGFR of <60 ml/min (RR 0.54; 95% CI: 0.2-1.42).
Conclusions: This meta-analysis suggests a potential benefit for statin use prior to angiography to reduce the incidence of CI-AKI. Additional research is needed to define better the benefits of statin therapy prior to angiography to prevent CI-AKI, especially in high-risk patients with chronic kidney disease who were largely underrepresented in the available trials.
Introduction
Contrast-induced acute kidney injury (CI-AKI) occurs commonly after iodinated contrast administration and is a major contributor to morbidity after cardiac angiography. CI-AKI is generally defined as an increase in serum creatinine concentration of >0.5 mg/dL or 25% above baseline within 48-72 hours after contrast administration1-4. The overall incidence of CI-AKI is less than 2% in a healthy population, but is as high as 50% in high-risk groups, including those with chronic renal impairment, diabetes, congestive heart failure, or advanced age1-4. Overall, CI-AKI accounts for 11% of in-hospital cases of acute kidney injury, is a significant predictor of mortality, and results in prolonged hospitalisation and increased healthcare costs5-7. The development of strategies to prevent CI-AKI is therefore critical.
Due to its pleiotropic effects, statin therapy has recently been suggested to be of potential benefit in preventing CI-AKI. Over the past several years, multiple randomised trials have been performed to examine this hypothesis: these trials have demonstrated varying benefit. As such, the current role for statin therapy in the prevention of CI-AKI remains unsettled. The purpose of this study was to perform a systematic review and a meta-analysis to define better the role of short-term statin use for the prevention of CI-AKI.
Methods
DATA SOURCES AND SEARCH STRATEGY
A systematic literature review of MEDLINE (1966 to week three of November 2013), Embase (1980 to November 2013), and the Cochrane CENTRAL Register (until the third quarter of 2013) was performed for randomised controlled trials investigating statin therapy for the prevention of CI-AKI8-26. The search was performed by combining various combinations of exploded versions of the Medical Subject Headings statins, contrast media, kidney failure (acute), and clinical trial, and the keywords nephropathy and prevention. In addition, we searched the reference list of identified relevant publications and reviewed abstracts of conference proceedings of the American Heart Association (AHA), American College of Cardiology (ACC), European Society of Cardiology (ESC), and Transcatheter Cardiovascular Therapeutics (TCT), and websites, including cardiosource.com, theheart.org, escardio.org, asn.org, and TCTMD.com for relevant material. No language, publication date, or publication status restrictions were imposed.
STUDY SELECTION
Two reviewers identified articles for further review by performing an initial screening of identified abstracts and titles. Studies identified by any reviewer were retained. Articles were considered for inclusion if they were randomised comparisons in which the treatment group received statin therapy for the prevention of CI-AKI in the adult population (age ≥18 years) undergoing invasive angiography. For studies not published in English, two reviewers fluent in the language of publication performed data abstraction. Discrepancies were resolved by consensus.
Included studies were of two types –those comparing high-dose to low-dose statin therapy and those comparing high-dose statin therapy to placebo. High-dose statin was defined as 80 mg of simvastatin, 40-80 mg of atorvastatin, or 10-40 mg of rosuvastatin. Low-dose statin was defined as 10-20 mg of simvastatin or atorvastatin (no trial used low-dose rosuvastatin). Studies were excluded if they included patients with end-stage renal disease or if event rates of CI-AKI were not reported.
DATA EXTRACTION AND QUALITY ASSESSMENT
Pre-specified data elements were extracted from each trial, including study size, design, inclusion/exclusion criteria, procedure type, type of contrast used, contrast volume, statin dosing protocol, country of origin, definition of CI-AKI, use of hydration, use of N-acetylcysteine, baseline renal function, incidence of CI-AKI, need for haemodialysis, death, myocardial infarction, length of hospital stay, and other study characteristics. Quality measures included the Jadad score and publication status27.
STATISTICAL ANALYSIS
From the abstracted data, we calculated the risk ratio for development of CI-AKI. The average effects for the outcomes and 95% confidence intervals (CIs) were obtained using a random-effects model as described by DerSimonian and Laird28. We chose the random-effects method because of its conservative summary estimate and incorporation of between and within study variance. To assess the heterogeneity of relative risks across trials, we used the Cochran’s Q statistical test, with a p-value <0.1 considered significant, and the I2 statistic. The I2 statistic was used to measure the consistency among trials with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively. Sensitivity analyses were performed to assess the effects of selected measures of study quality and clinical factors. Summary estimates were calculated for select subgroups.
A funnel plot was used to assess the presence of small study effects and other reporting biases by plotting the standard error against the log risk ratio. Using Egger’s linear regression method and Begg’s rank correlation test, we examined the association between study size and estimated treatment effects, with a p-value <0.1 considered significant. Publication bias was further assessed by applying the trim-and-fill method, which aims to identify and correct for funnel plot asymmetry. The influence of each study on the overall Q-test for heterogeneity was assessed by creating a Baujat plot. Based on this, a leave-one-out analysis was performed in order to identify significant changes in the summary estimate and I2 statistic after removing individual studies.
The p-value threshold for statistical significance was set at 0.05 for effect sizes. Analyses were conducted by S.S.B. in R (version 3.0.2) using the metafor package (version 1.9). The study was performed in accordance with the recommendations set forth by the Quality of Reporting of Meta-Analyses (QUOROM) workgroup29.
Results
ELIGIBLE STUDIES
There were 483 potentially relevant articles identified from the initial electronic literature search (Figure 1). After title review, 64 citations were identified as relevant to the focus of the study and were manually reviewed. Nineteen randomised controlled trials were identified. Five of these RCTs did not meet our inclusion criteria and were excluded (two did not report CI-AKI event rates, one included patients with ESRD, one had an inappropriate control, and one evaluated coronary computed tomography angiography). The study by Quintavalle was a post hoc subgroup analysis of an RCT: because patients were analysed according to their initial randomisation assignment, this trial was included in the analysis10. Review of references of relevant articles and conference proceedings led to the identification of five additional RCTs that met inclusion criteria and were also included in the analysis. Thus, a total of 19 RCTs were included8-26.
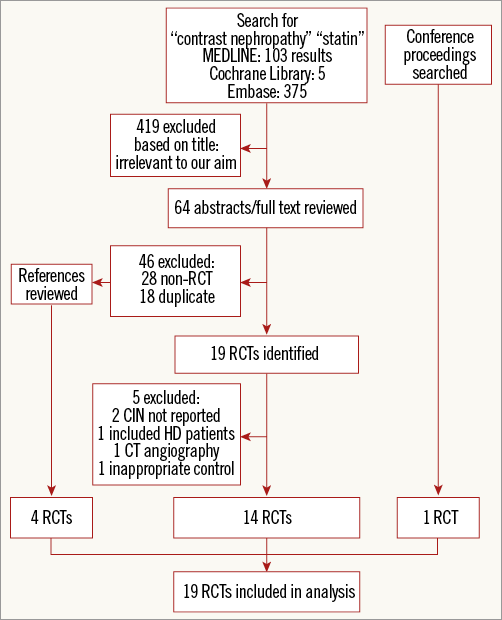
Figure 1. Study flow. Flow chart of meta-analysis. CIN: contrast-induced nephropathy; HD: haemodialysis; RCT: randomised controlled trial
The characteristics of the 19 RCTs are summarised in Table 1 and Table 2. There was a total of 7,161 patients (3,499 patients in the treatment arm and 3,662 patients in the control arm). Indication for angiography was diagnostic coronary angiography or PCI in all trials except for one trial, which included patients undergoing cerebral arterial intervention. Seven studies compared high-dose statin to low-dose statin, and 12 studies compared high-dose statin to placebo or no therapy. The duration of treatment with statin prior to angiography ranged widely among studies (two hours to seven days), with 10 trials beginning treatment 0-1 day prior to angiography and nine trials beginning treatment two to three days prior to angiography.
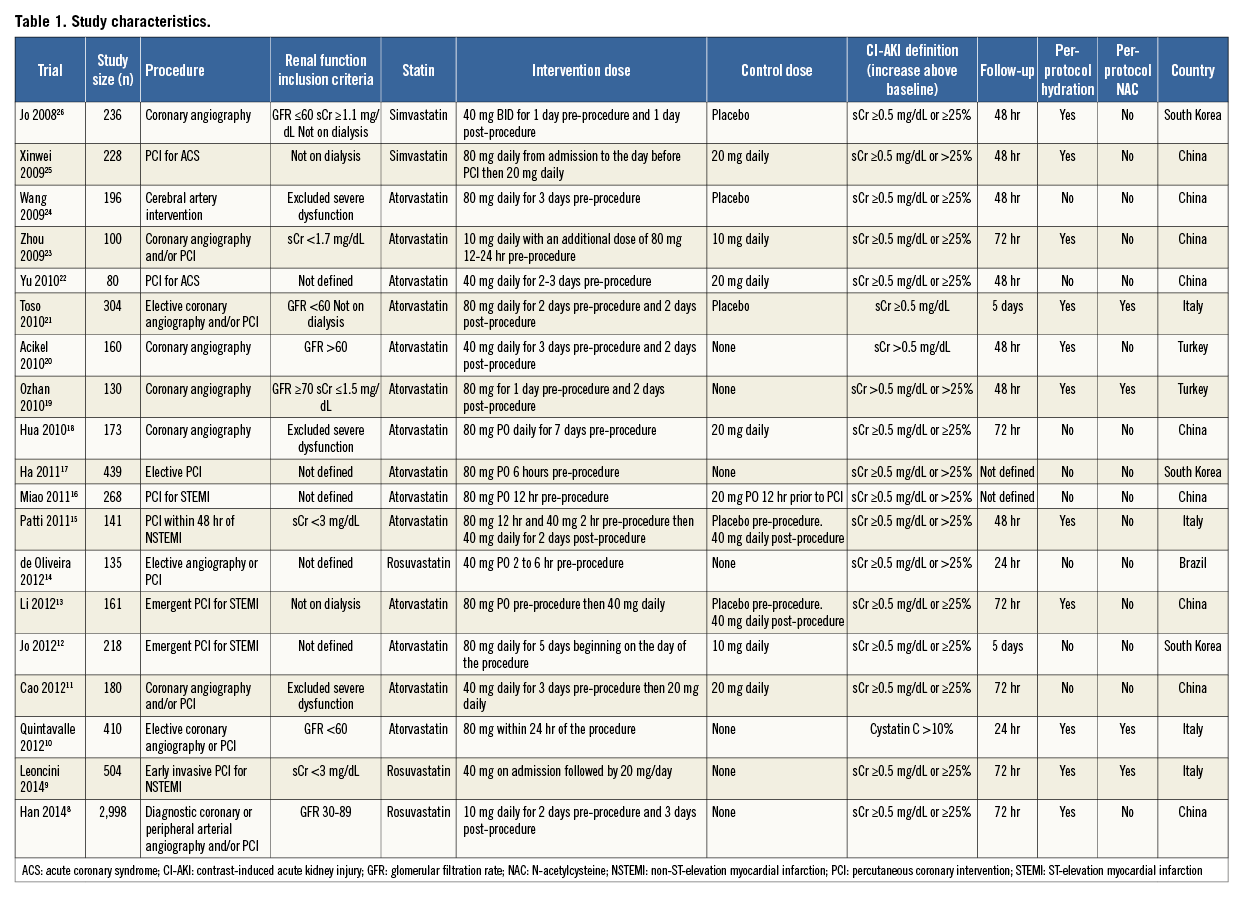
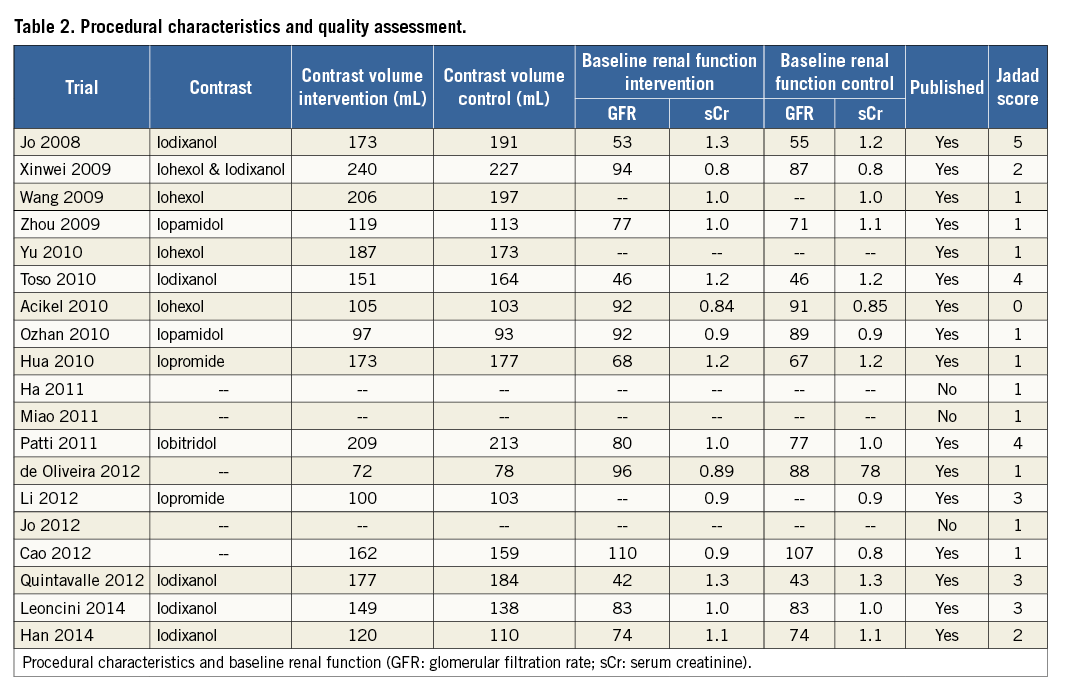
The duration of follow-up for the primary event rate of CI-AKI ranged from 24 hours to five days. Mean baseline eGFR was <60 mL/min/1.73 m2 in three studies, >60 mL/min/1.73 m2 in 12 studies, and not reported in four studies. There were 13 reports in English, five in Chinese, and one in Portuguese.
CI-AKI PREVENTION
In total, there were 138 cases of CI-AKI in the statin treatment group and 289 cases of CI-AKI in the control group (overall incidence 4.0% vs. 7.9%). The analysis demonstrated a significant reduction in the incidence of CI-AKI in patients treated with statin prior to invasive angiography when compared with control (RR 0.52; 95% CI: 0.40-0.67) (Figure 2).
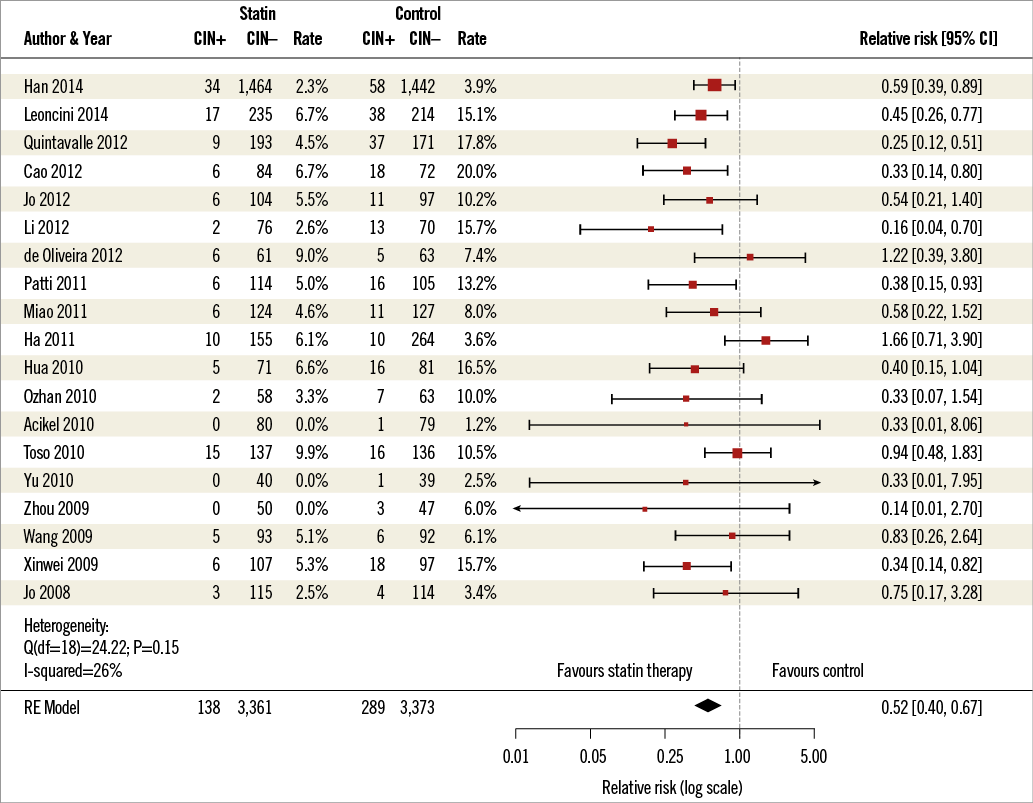
Figure 2. Forest plot. Forest plot of randomised trials meeting inclusion criteria. CI-AKI: contrast-induced acute kidney injury; RE: random effects
The benefit of statin therapy remained significant across many subgroups (Figure 3). The protective effect of statin therapy in preventing CI-AKI was present in trials where the control group received low-dose statin, placebo, or no therapy. This benefit was also independent of mean contrast volume, contrast type, geographic origin of study, or use of N-acetylcysteine. The benefit was seen with all three statin types evaluated (atorvastatin, rosuvastatin, and simvastatin), and was also independent of duration of statin exposure prior to angiography. Patients in whom the indication for coronary angiography was an acute coronary syndrome had significant benefit from pre-treatment with statin therapy, whereas those who underwent angiography for non-acute coronary syndromes did not.
Studies which enrolled a patient population with a mean eGFR ≥60 mL/min/1.73 m2 demonstrated a significant reduction in CI-AKI with statin therapy (RR 0.47; 95% CI: 0.37-0.61), whereas studies which included a patient population with a mean eGFR of <60 mL/min/1.73 m2 (RR 0.64; 95% CI: 0.20-1.42) did not. We further assessed the influence of baseline renal function on outcomes by identifying event rates for patients with CKD stage 3 or worse. Based on trial inclusion criteria or from reported subgroup data, event rates were available for 1,397 patients with an eGFR <60 mL/min/1.73 m2 and 2,944 patients with an eGFR >60 mL/min/1.73 m2. In this analysis, statin therapy was protective against the development of CI-AKI in both groups (Figure 3).
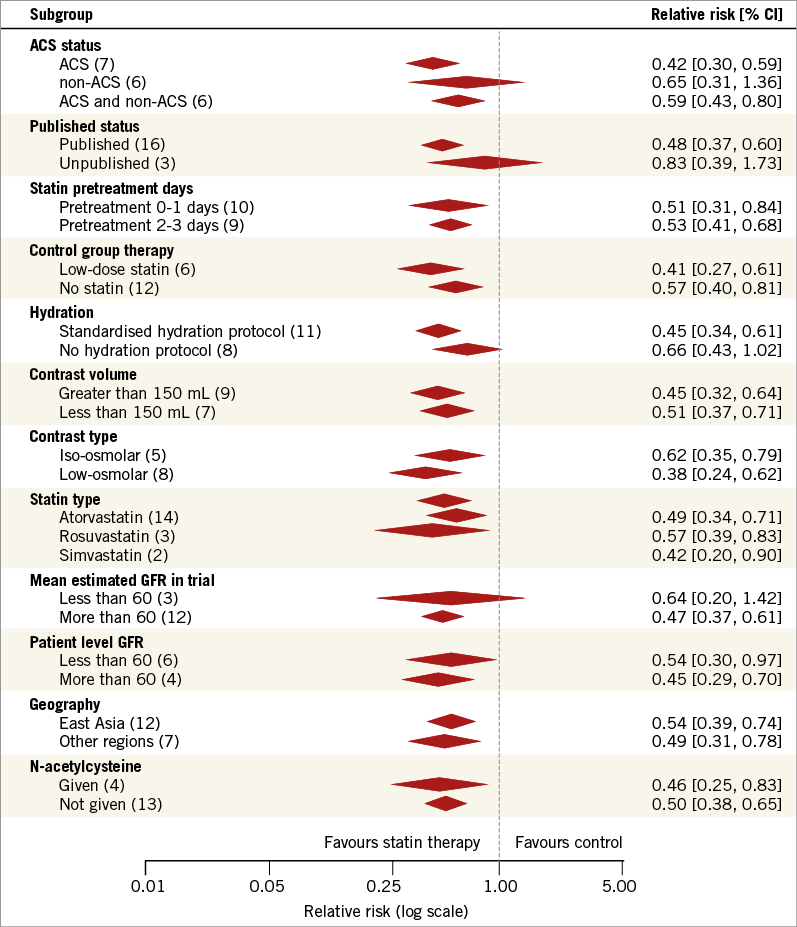
Figure 3. Subgroup analysis. Forest plot of summary estimates from select subgroups. eGFR: estimated glomerular filtration rate
Few studies reported the rates of death (n=3), myocardial infarction (n=2), or the need for haemodialysis (n=5); however, these event rates were similar between statin and control groups (0.3% vs. 0.4%, 1.3% vs. 3.3%, and 0% vs. 0.3%, respectively) (Online Appendix).
SMALL STUDY BIAS AND INFLUENCE ANALYSIS
Visual inspection of the funnel plots (Figure 4) did not suggest a small study effect for the overall analysis, and regression and rank correlation tests were not statistically significant for a small study effect or publication bias (p=0.45 and p=0.95, respectively).
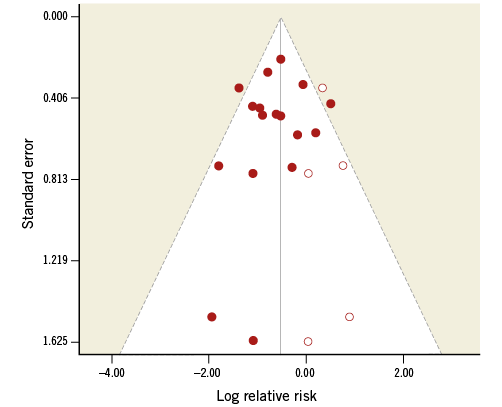
Figure 4. Funnel plot. Solid circles represent original studies. Open circles represent hypothetical or imputed studies.
Review of the Baujat plot (Online Appendix) demonstrated that three trials (Ha 2011, Quintavalle 2012, Toso 2010) provided the greatest contribution to the overall heterogeneity and influence on overall study results. The influence of each study on the overall treatment effect and heterogeneity was determined by deleting each in turn from the analysis and noting the degree to which the pooled effect size changed. Elimination of any one trial had no significant influence on the results (Online Appendix).
MEASURES OF QUALITY
The overall quality of the trials was low (mean Jadad score 1.9±1.4) and did not change after elimination of the three unpublished trials (Table 2). Eleven trials had a score of 0-1, while only three trials had a score of 4-5. The most common reason for low Jadad scores was lack of placebo/blinding (15 trials) and failure to describe withdrawals and drop-out (16 trials).
Discussion
We performed a systematic review and meta-analysis which compared high-dose statin to control for the prevention of CI-AKI after angiography. This represents the largest meta-analysis of its kind and is unique because we did not exclude studies based on country of origin, language of publication, or publication status. This significantly reduces the risk of bias in our study.
We identified 19 RCTs including 7,161 patients. The pooled effect of these trials suggests that statin therapy prior to angiography may reduce the incidence of CI-AKI. Similar effects were seen across multiple subgroups. Although more than half of the randomised trials were conducted in East Asia, a benefit with statin therapy was seen across Asian and non-Asian subgroups, suggesting that the results may be applicable to a wide variety of patient populations and ethnicities. Statins were protective against CI-AKI independent of contrast type and volume used during angiography. In the subgroup analysis, a similar protective benefit was seen with all three statins evaluated (rosuvastatin, atorvastatin, or simvastatin). The duration of statin treatment prior to contrast exposure ranged from less than two days to two to three days prior to angiography, and clinical benefit with statin therapy was seen independent of pre-treatment duration. Too few trials reported the incidence of death, myocardial infarction, or the need for subsequent dialysis to draw meaningful conclusions about these endpoints.
The mechanism by which statins may protect against iodinated contrast renal injury is not entirely clear. Statins may act to prevent CI-AKI by preserving endothelial function through enhancement of vasodilator and attenuation of vasoconstrictor activity in vascular walls30. For example, statins have been shown to prevent hypoxia-induced downregulation of endothelial nitric oxide synthase, leading to increased nitric oxide production30,31. Statin-induced downregulation of angiotensin receptors and decreased endothelin synthesis may also limit contrast-induced renal hypoxia32. Statins may also counteract contrast-induced oxidative stress by limiting ROS formation30,31,33. Statins also have anti-inflammatory properties, which have been demonstrated in rat models and large clinical trials34,35. Despite these postulated effects, the actual mechanism by which statins may lessen the risk of contrast nephropathy remains largely unknown and further research is needed to understand this better.
Despite the lack of significant statistical heterogeneity in the primary findings, clinical heterogeneity among trials should be taken into consideration in the interpretation of our findings. Hydration protocols differed among studies and per-protocol hydration was not required in one third of the trials. Because periprocedural hydration has been shown to reduce the risk of CI-AKI significantly and is the current standard of care for at-risk patients, trials evaluating new therapies for the prevention of CI-AKI are required to demonstrate a benefit that is incremental to adequate periprocedural hydration6. That many trials did not standardise periprocedural hydration is of concern.
Patients with estimated eGFR <60 mL/min/1.73 m2, who are at highest risk from CI-AKI and most in need of preventative therapy, were largely underrepresented in these trials. Only three trials enrolled a patient population with a mean eGFR <60 mL/min/1.73 m2. Because of this, there are insufficient trial data to determine reliably whether patients with CKD benefit from statin therapy prior to contrast exposure. Data from the two largest RCTs reported conflicting results. A significant reduction in the incidence of CI-AKI was observed in the subgroup of 294 patients with a baseline creatinine clearance <60 mL/min/1.73 m2 in the trial by Leoncini et al (OR 0.35; 95% CI: 0.15-0.81; p=0.014), whereas no benefit was seen in the subgroup of 450 patients with eGFR 30-60 mL/min/1.73 m2 in the trial by Han et al (OR 0.81; 95% CI: 0.32-2.10; p=0.67)8,9. Furthermore, no significant benefit with statin therapy was seen in our pooled analysis of the three trials which enrolled a population with a mean eGFR of <60 mL/min/1.73 m2 (RR 0.54; 95% CI: 0.2-1.42).
Another source of clinical heterogeneity is the variance in the baseline rates of CI-AKI observed in the studies, many of which were unusually high given the relatively low-risk patients enrolled in these trials. Because the incidence of CI-AKI among patients with estimated eGFR ≥60 mL/min/1.73 m2 is quite low (<5-10%)1-4, trials which enrolled patients with eGFR ≥60 mL/min/1.73 m2 would be expected to have difficulty generating a sufficient number of events to detect a significant treatment effect with statin therapy. Despite this, there were six trials which enrolled low-risk patients (mean eGFR 67-107 mL/min/1.73 m2) and reported unusually high event rates in the control group, ranging from 10%-20%9,11,13,15,18,25. This higher than predicted event rate in the control group when compared to the treatment group raises concerns about the quality and reliability of the studies.
Conclusions
In the meta-analysis presented here, we detected substantial benefit with the use of high-dose statin therapy prior to angiography for the prevention of CI-AKI and the benefit continued to be seen across multiple subgroups. Despite these robust findings, our data are limited by significant clinical heterogeneity among trials, including lack of routine periprocedural hydration, underrepresentation of patients with significant renal dysfunction, unusually high CI-AKI rates in the control groups of many trials, and overall poor study quality as assessed by the Jadad score. Therefore, we believe that there is insufficient evidence to recommend routine high-dose statin therapy for the prevention of CI-AKI, especially in patients with eGFR <60 mL/min/1.73 m2 where data are limited. Defining the role of statin therapy better in the prevention of CI-AKI will require a large prospective randomised controlled trial which includes patients with eGFR <60 mL/min/1.73 m2 , and which utilises a per-protocol hydration scheme.
| Impact on daily practice The use of statin therapy prior to angiography for the prevention of CI-AKI has been evaluated in numerous RCTs and remains a topic of debate. In this meta-analysis of 19 RCTs we identified a significant reduction in the incidence of CI-AKI in patients treated with statins prior to invasive angiography when compared with control. Despite this, due to significant limitations of the available randomised trials, we believe that there is insufficient evidence to recommend routine high-dose statin therapy for the prevention of CI-AKI in daily practice, especially in patients with eGFR <60 mL/min/1.73m2 where data are limited. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data

