
Abstract
Aims: Many studies have analysed the occurrence of acute kidney injury (AKI) after percutaneous coronary intervention (PCI) but there are limited data relating to AKI risk in patients undergoing carotid artery stenting (CAS). The aim of this study was to determine the incidence and predictors of AKI in patients undergoing proximal protected CAS.
Methods and results: We analysed 456 patients undergoing proximal protected CAS. A binomial multivariate logistic model was developed including patients’ clinical and angiographic/procedural characteristics. AKI (defined as an sCr increase ≥0.3 mg/dl or ≥1.5-fold sCr increase from baseline or more than 50% increase from baseline, within 48 hours post procedure) occurred in 155 patients (34%). AKI patients were more frequently affected by hypertension, diabetes, dyslipidaemia and anaemia, and presented lower renal function at baseline. Higher contrast volume to creatinine clearance ratio (2.40±1.44 vs. 2.08±1.15; p=0.01), lower post-procedural mean arterial pressure (MAP) (94.3±17.7 vs. 99.6±18.5 mmHg; p=0.003) and a more frequent post-procedural systolic pressure drop (∆SBP >50 mmHg) (23.9% vs. 14.3%, p=0.01) were observed in the AKI group of patients. At multivariate analysis, independent predictors of AKI were ∆SBP >50 mmHg, diabetes mellitus and dyslipidaemia.
Conclusions: AKI can occur quite frequently after proximal protected CAS and is related to clinical and procedural features. These data should be confirmed in larger registries or randomised trials.
Abbreviations
AKI: acute kidney injury
CAS: carotid artery stenting
CCA: common carotid artery
CEA: carotid endarterectomy
ECA: external carotid artery
EPD: embolic protection device
ICA: internal carotid artery
PCI: percutaneous coronary intervention
Introduction
Carotid artery stenting (CAS) is considered to be a reasonable alternative to carotid endarterectomy (CEA), particularly in patients at high risk for CEA1. Expert consensus suggests that the use of embolic protection devices (EPD) reduces the risk of stroke during CAS1. Among the EPD that are in clinical use, proximal EPD have the theoretical advantage of providing embolic protection during all phases of the intervention2 and are associated with a reduced occurrence of post-procedural cerebral new ischaemic lesions, when compared to distal protection devices3.
Post-CAS acute kidney injury (AKI) can have a variety of causes, such as the administration of iodinated contrast media that could injure the renal tubular cells, hypotension that decreases in renal perfusion or atheroembolism that can result in kidney ischaemia4.
Although much has been published concerning the epidemiology of AKI in the setting of coronary and other cardiac interventions, there are limited data dealing with AKI risk in patients undergoing distal protected CAS5; no data are available for patients undergoing proximal protected CAS.
The aim of this study was to evaluate the incidence and predictors of AKI in patients undergoing proximal protected CAS.
Methods
PATIENT POPULATION
From January 2013 to March 2015, 456 consecutive patients undergoing CAS using proximal EPD at our institution were prospectively enrolled in this registry.
Inclusion criteria included the degree of internal carotid artery (ICA) stenosis, determined by angiography according to the North American Symptomatic Carotid Endarterectomy Trial Criteria: 1) asymptomatic stenosis >80%, and 2) symptomatic stenosis >50%6.
Symptomatic was defined as a carotid stenosis associated, within six months before the intervention, with amaurosis fugax, ipsilateral hemispheric transient ischaemic attack, or ipsilateral ischaemic stroke not resulting in a major residual neurological deficit (stroke scales: Barthel score ≤60, National Institutes of Health Stroke Scale score ≥15, or Rankin Scale score >3)7.
Patients with the following criteria were excluded7: presence of a critical stenosis of the ipsilateral common carotid artery (CCA), contraindication to thienopyridines, recent (<72 hrs) administration of iodinated contrast, refusal to provide informed consent before enrolment.
Smokers included current and former smokers. Hypertension was diagnosed if the systolic arterial pressure was >140 mmHg and/or diastolic arterial pressure was >90 mmHg on repeated measurements or if the patient used antihypertensive drugs8. Hypercholesterolaemia was diagnosed if plasma total cholesterol was >200 mg/dl, plasma low-density lipoprotein cholesterol was >130 mg/dl, or if the patient used lipid-lowering drugs because of a history of hypercholesterolaemia8. Diabetes mellitus was diagnosed if plasma fasting glucose was >126 mg/dl or if the patient used hypoglycaemic agents8. Coronary artery disease was defined as history of previous disease or presence of coronary obstruction during angiography during the same admission. Estimated glomerular filtration rate (eGFR) was calculated applying the Modification of Diet in Renal Disease formula9, and moderate-to-severe chronic kidney disease (CKD) was diagnosed if it was <60 ml/min/1.73 m2.
CAS PROCEDURE
All procedures were performed percutaneously with the patient under local anaesthesia. Heart rate and blood pressure were assessed continuously during the procedure, which has been described in detail previously6. The Mo.Ma™ system (Medtronic, Minneapolis, MN, USA) was adopted as proximal EPD. Only self-expanding carotid stents were deployed (Carotid WALLSTENT™ [Boston Scientific, Marlborough, MA, USA], Xact® [Abbott Vascular, Santa Clara, CA, USA], PRECISE® [Cordis, Cardinal Health, Milpitas, CA, USA], Acculink® [Abbott Vascular], and Cristallo Ideale™ [Medtronic]).
During the procedure, only non-ionic contrast media was used, either low-osmolar contrast (Iomeron® [Bracco Diagnostics Inc., Monroe Township, NJ, USA], Omnipaque™ [GE Healthcare, Waukesha, WI, USA], Ultravist [Schering AG, Berlin, Germany], Xenetix® [Guerbet, Villepinte, France]), or an iso-osmolar one (Visipaque [GE Healthcare]).
Blood pressure was measured non-invasively before and after the procedure, when the patient was in the ward. An invasive blood pressure monitoring was adopted during the procedure and until the patient was in the catheterisation laboratory.
CONCOMITANT THERAPY
All patients received aspirin (75 to 160 mg/day) and should have been on ticlopidine (250 mg twice daily) for at least seven days. Alternatively, patients received a clopidogrel preload (300 mg) 24 hours before the procedure. After the procedure, thienopyridines were continued for at least three months, whereas aspirin was prescribed lifelong.
For anticoagulation, 70 to 100 IU/kg of heparin was administered before wiring the external carotid artery (ECA), with the intention of achieving an activated clotting time (ACT) of >250 s. Additional heparin was administered at the operator’s discretion according to ACT values10.
As prophylactic therapy for AKI prevention, all patients were pre-treated with fluid infusion (NaCL 0.9% 1 ml/kg/hr) at least in the 24 hours before the procedure11 and for at least 24 hours post procedure. During the procedure, they received atropine before stent post-dilation in order to prevent the occurrence of carotid sinus manipulation-induced bradycardia and hypotension.
POST-PROCEDURAL PATIENT MANAGEMENT AND FOLLOW-UP
Femoral sheaths were removed when the ACT was <150 s. Access-site haemostasis was achieved by manual compression in all patients.
Serum creatinine was measured the day before procedure and post procedure (24 and 48 hours). In those patients in whom a non-AKI diagnostic increase in serum creatinine was detected at 48 hours, an additional serum creatinine measurement was performed at 72 hours. The higher creatinine value detected among post-procedural measurements was considered the creatinine peak. A complete blood count was obtained before the CAS procedure and before hospital discharge.
All patients received a follow-up clinical assessment at one month. Clinical examination assessed overall general condition, neurological signs and symptoms, medications, hospitalisations, or any type of complication that occurred after the procedure.
CLINICAL OUTCOME
Acute kidney injury was defined as an sCr increase ≥0.3 mg/dl (≥26.4 μmol/l) or a ≥1.5-fold sCr increase from baseline or more than 50% increase from baseline12, within 48 hours post procedure.
DEFINITIONS
The primary endpoint of the study was the incidence of AKI post procedure.
Secondary endpoints were the incidence of major adverse cardiovascular and cerebrovascular events (MACCE), including death, myocardial infarction, and any stroke in-hospital and at 30 days.
Occlusion intolerance (OI) was defined as any transient neurological deficit observed during occlusion time but showing a complete recovery within 20 minutes after restoring antegrade flow7.
Occlusion time (time of flow blockage) was defined as the time from the inflation to the deflation of the proximal balloon in the ICA7. Technical success was defined as the ability to implant a carotid stent successfully with a residual ICA stenosis <30%7. Procedural success was defined as technical success without the occurrence of MACCE7.
Neurological complications were classified as one of the following: 1) minor stroke, defined as a new neurological deficit that resolved completely within 30 days or an increase in the National Institutes of Health Stroke Scale score of >3; and 2) major stroke, defined as a new neurological deficit that persisted for >30 days or an increase in the National Institutes of Health Stroke Scale score of >4 13.
High surgical procedural risk was defined as the presence of any of the following: clinically significant cardiac disease, severe pulmonary disease, contralateral ICA occlusion, contralateral recurrent laryngeal nerve palsy, previous radical neck surgery or radiotherapy, recurrent stenosis after CEA and age >80 years7.
Patients were considered at high surgical risk if they presented with at least ≥1 high surgical procedural risk criterion1.
Anaemia was defined as haematocrit value <39% in males and <36% in females.
Haemodynamic depression was considered present if systolic blood pressure decreased below 90 mmHg during or within 24 hours after the stent implantation procedure14.
Post-procedural systolic pressure drop (∆SBP) was considered relevant if >50 mmHg 5.
Maximum contrast dose was defined as five times body weight (kg) divided by the serum creatinine value5.
Contrast ratio was defined as the ratio between the actual volume of contrast received by the patient and the maximum contrast dose5. High contrast volume was defined as a contrast ratio >1 5.
STATISTICAL ANALYSIS
Statistical analyses were performed using SPSS, Version 23.0 (IBM Corp., Armonk, NY, USA). Variables were expressed as absolute numbers and percentages or mean±SD. Comparisons between groups were made with the t-test for unpaired samples or the chi-square test when appropriate. Univariate analysis was performed to verify independent associations between baseline characteristics and AKI development. Significant associations at univariate analysis were included in a binomial multivariate logistic model.
Interaction analysis was performed to evaluate consistency of the multivariate model and independent associations. For all the analyses, a p-value ≤0.05 was considered statistically significant.
Results
The patient population enrolled (N=456) in this study exhibited a robust prevalence of cardiovascular risk factors and almost half of the patients were at high surgical risk for CEA (n=226; 49.6%). Almost one fifth of the patients underwent CAS due to symptomatic cerebrovascular disease (N=82; 18%). A moderate-to-severe chronic kidney disease (CKD = eGFR <60) was evident in 107 patients (23.5%).
Contrast media type use, either low-osmolar or iso-osmolar, was comparable between patients with AKI and patients without AKI.
A mean dose of 130 ml of contrast media was administered which resulted in a mean contrast volume/CrCl ratio of 2.2; only in 45 patients did the ratio exceed 3.7, and only seven patients received a high contrast load (1.5%).
Technical success was achieved in all patients. Occlusion intolerance occurred in 138 patients (30.3%) while haemodynamic depression occurred in 33 patients (7.2%). No patients died during the hospital stay and one patient had a minor stroke. The cumulative incidence of death and stroke was 0.2%; consequently, the procedural success rate was 99.8%.
Serum creatinine was detected in every patient up to 48 hours post procedure. AKI occurred in 141 patients at 24 hours (30.9%), and in 155 patients (34%) at 48 hours. In those patients in whom a non-AKI diagnostic rise in serum creatinine was detected at 48 hours, an additional serum creatinine measurement was performed at 72 hours. No additional patients experienced AKI between 48 and 72 hours post procedure.
The incidence of death and stroke in patients experiencing AKI post procedure (Table 1) was negligible and comparable with the rate observed in patients who did not experience a significant post-CAS serum creatinine increase (p=0.47).
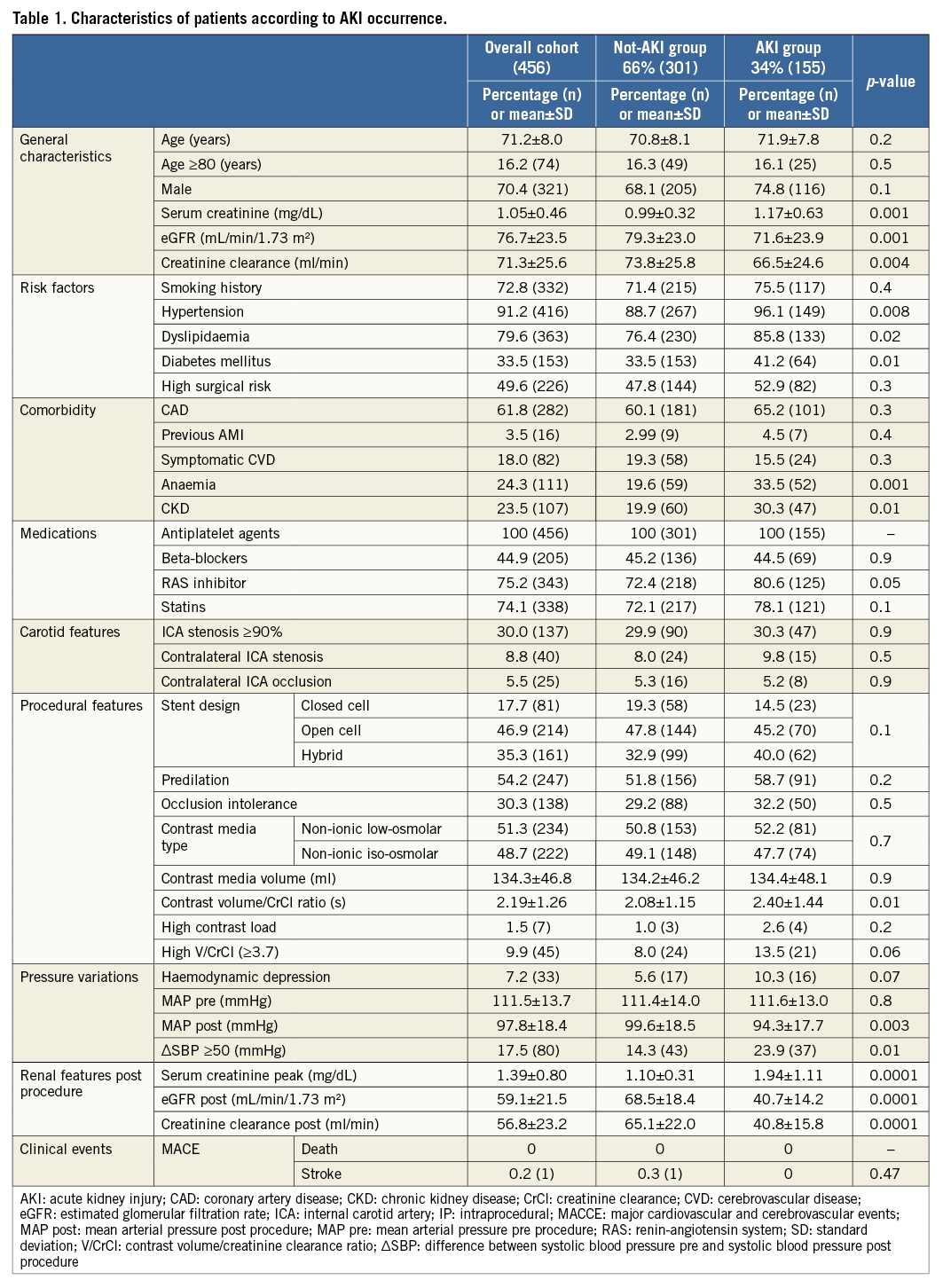
We divided the patients into two groups: one group included those who developed AKI and the other group those who did not (Table 1). Notably, patients in the AKI group presented higher baseline serum creatinine values, lower eGFR and creatinine clearance, were more frequently hypertensive, dyslipidaemic, and anaemic, and had CKD and/or diabetes. A higher contrast volume to creatinine clearance ratio was observed in the patients who had AKI.
Haemodynamic assessment demonstrated lower post-procedural mean arterial pressure (MAP) and a more frequent post-procedural systolic pressure drop (∆SBP >50 mmHg)5 in the group of patients experiencing AKI.
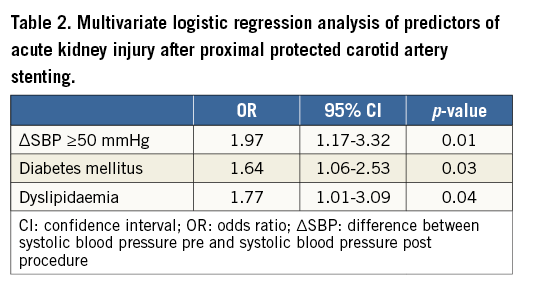
The multivariate logistic regression analysis evaluating predictors of AKI is shown in Table 2. Among the statistically significant predictors, the most powerful independent predictor of AKI was ∆SBP >50 mmHg. The presence of diabetes mellitus and dyslipidaemia also indicates a significantly higher risk of AKI development. No statistically significant interaction effect among variables included in the multivariate analysis was apparent.
Discussion
This study demonstrates that the occurrence of AKI after proximal protected CAS15 is a frequent post-procedural complication that is independently predicted by a systolic post-procedural pressure drop >50 mmHg as well as by the presence of diabetes and/or dyslipidaemia.
Contemporary outcomes reported for almost a million consecutive patients undergoing percutaneous coronary intervention (PCI) at 1,253 sites participating in the National Cardiovascular Data Registry (NCDR) CathPCI registry indicated an overall rate of contrast-induced acute kidney injury (CI-AKI) of 7.1%16. Little is known about the risks of AKI in patients undergoing CAS. Recently, it has been reported that, out of 126 patients5 with pre-existing CKD undergoing distal protected CAS, AKI occurred in 17% of the cases. This complication could be predicted by the occurrence of haemodynamic depression (mostly due to hypotension) and not by the amount of contrast media administered5. This study refers only to patients with CKD5; no data are available on the role of haemodynamic depression and contrast media volume administration in the occurrence of AKI in non-CKD patients undergoing CAS.
In our experience, we have observed the highest rate of AKI occurrence ever reported (34%). At this time, we cannot provide a definitive explanation for this finding. Comparing our study to the above-mentioned study5, it is possible to identify some differences: administration of a slightly different amount of contrast media, the use of different prophylaxis strategies and the use of different EPD. In particular, the difference in contrast load is too small to consider this difference as a possible explanation for the different AKI incidence. It should also be noted that in the previous study5 contrast volume administration did not predict AKI occurrence. Regarding prophylaxis strategies, it should be borne in mind that, while in the study from Donahue et al a multifactorial strategy was adopted5 (hydration with sodium bicarbonate solution in patients with eGFR 30 to 59 ml/min/1.73 m2 or hydration with normal saline plus NAC controlled by the RenalGuard® system [RenalGuard Solutions Inc., Milford, MA, USA] in patients with eGFR <30 ml/min/1.73 m2), in our study all patients were pre-treated only by hydration with NaCL 0.9% 1 ml/kg/hr.
In the light of the recent results of the AMACING trial17, which found no prophylaxis to be non-inferior to prophylactic intravenous hydration in the prevention of contrast-induced nephropathy, the prophylaxis adopted in our study can be claimed to be insufficient to prevent AKI and partially responsible for the higher AKI incidence observed in our study.
One further factor that can affect AKI occurrence is the use of proximal EPD, which requires the manipulation of larger catheters in the aorta. This can result in a higher risk of peripheral embolisation which, if it occurs in the kidney, can lead to a post-procedural reduction of the renal function. This may also have accounted for the higher observed incidence of AKI.
In our report, the group of patients experiencing AKI had a worse baseline renal function and was more frequently affected by CKD; despite this, none of the conditions resulted in being an independent AKI predictor. Similarly, the type of contrast (iso-osmolar or low-osmolar) did not affect the rate of AKI occurrence. Contrast volume administered was comparable in the two groups; however, due to a lower average renal function in the AKI group, the ratio between contrast volume and creatinine clearance was higher in the group of patients which experienced AKI. Nevertheless, neither absolute volume nor contrast volume/CrCl ratio resulted in being an independent predictor of AKI. This confirms the data available in patients with CKD undergoing distally protected CAS5 and underlines the necessity to understand better the actual relationship between contrast agents and AKI onset, at least in the setting of patients undergoing CAS. In the current literature, the relationship between contrast application and AKI remains controversial. Recent controlled studies have demonstrated that contrast media represents an AKI risk factor only in patients with a significant renal impairment18,19.
A contrast volume/CrCl ratio >3.7 is a well-known independent predictor of increased creatinine value after PCI. We investigated whether the same ratio could be useful in case of proximally protected CAS. Despite the fact that a contrast volume/CrCl ratio >3.7 was twice as frequent in the AKI group, this difference did not match the statistically relevant value, maybe due to the limited sample size of the study population. Thus, this finding should be further investigated in larger studies.
Finally, our study suggests that the operator must pay a great deal of attention to procedural details. If there is a systolic pressure drop >50 mmHg, the chance of having AKI is relatively high; therefore, the operator should be aware of this possibility and be ready to manage this haemodynamic depression. Avoidance of bradycardia and hypotension is an important part of the management of patients during CAS. This can be achieved by pre-treatment with atropine and cessation of antihypertensive drugs and phosphodiesterase inhibitors on the day of the procedure.
As previously suggested4, in order to manage the occurrence of hypotension or bradycardia promptly, intravenous dopamine should be prepared ready for infusion and isoproterenol should be available if bradycardia occurs despite anticholinergic pre-medication. If hypotension occurs, volume administration can also be used, with care not to overhydrate, which could lead to bladder distension and reflex vagal stimulation5.
To our knowledge there are no data in the literature supportive of this proposed strategy to prevent CAS-related haemodynamic depression. This could perhaps be considered the topic for future investigational studies.
Study limitations
The most important limitations are the size of the study population, which limits the power of multivariable logistic regression, and the topic itself, which deals with a group characterised by impaired kidney function, advanced age, higher cardiovascular risk profile, more frequent haemodynamic instability and higher contrast media volume. Absence of warranted periprocedural and post-procedural hydration should also be considered among the study limitations. Finally, the absence of serum creatinine collection at 30 days represents another noteworthy limitation because we do not know how many patients had recovered from renal dysfunction at that time point.
Conclusions
Our results suggest that the incidence of AKI in this category of patient is quite frequent. Diabetes and dyslipidaemia, as well as systolic pressure drop, represent independent predictors of AKI. The volume of contrast media administered does not seem to predict post-procedural AKI occurrence. The study findings should be considered hypothesis-generating ahead of a clinical trial.
| Impact on daily practice The occurrence of AKI after proximal protected CAS is quite frequent and seems to be more common in patients with diabetes and/or hypercholesterolaemia. The occurrence of a relevant reduction of post-procedural systolic pressure also increases the risk of AKI. CAS operators should be aware of this information in order to prevent and, eventually, promptly manage (i.e., fluid infusion, atropine or dopamine administration) this event. |
Acknowledgements
F. Iacovelli is currently attending the Cardiopath PhD programme at the University of Naples “Federico II”.
Funding
Institutional internal funding.
Conflict of interest statement
The authors have no conflicts of interest to declare.

