Abstract
Aims: We aimed to determine the success, safety and long-term durability of carotid artery stenting (CAS) in stroke prevention for all-comers managed with mandatory neuroprotection and a tailored-approach to intervention.
Methods and results: From our CAS registry (beginning July 1997) all procedures up to September 2007 with intention-to-treat by stenting under distal filter or proximal occlusion neuroprotection devices were analysed (N=1523; mean age 72 years [237 ≥80 years, 15.5%]). Indications included symptomatic stenoses ≥50% (366, 24.1%) and asymptomatic stenoses ≥80% (1157, 75.9%). CAS success was 99.6% and the 30-day all-stroke/death rate was 1.5% (minor stroke 11 [0.7%], major stroke 8 [0.5%], death 5 [0.3%]). The risk was 1.2% for asymptomatic patients and 2.7% for symptomatic patients (p=0.042). Regarding octogenarians this risk was 2.1% versus 1.5% for patients ≤79 years (p=0.47). Symptomatic octogenarians had a higher risk than other groups (OR 3.9, 95% CI 1.06 to 14.0): asymptomatic ≤79 1.2%, asymptomatic ≥80 1.2%, symptomatic ≤79 2.3% and symptomatic ≥80 4.5%. The event free survival rates from all strokes or stroke-related deaths at eight years were 96% for asymptomatic and 92% for symptomatic patients.
Conclusions: Results from this large cohort show that carotid stenting in a real-world setting is safe and efficacious, and durable in the long-term prevention of stroke.
Introduction
Carotid endarterectomy (CEA) has been shown to be effective in the long-term prevention of stroke for symptomatic and asymptomatic patients with obstructive carotid disease.1-4 Carotid artery angioplasty and stenting (CAS) has an important role due to its potential as complimentary therapy for patients considered high-risk for CEA, and as an alternative choice for a large subset of patients because it is a less invasive revascularisation procedure.
Evidence is accumulating from registry-data that CAS is producing promising immediate and intermediate results.5-9 Randomised controlled trials (RCTs) comparing both therapies have been plagued with difficulties in enrolment, results have been conflicting, criticisms have been levied against trial designs especially with regard to procedural protocols and expertise; and hence a clear consensus cannot be established.10-17 Also, there is a paucity of data regarding the long-term durability of CAS beyond 5 years in the prevention of stroke.18-22 Hence, we conducted an analytical observational study to assess the outcomes of a cohort of patients; with the purpose to determine, firstly, the success and safety of CAS for all-comers in a “real-world” setting managed with a “tailored-approach”23,24 and a mandatory use of embolic protection devices (EPDs)25; and, secondly, to determine the long-term durability of CAS in preventing stroke.
Methods
Tailored-approach to CAS
The Cardio-Angiology Unit at Villa Maria Cecilia Hospital, Cotignola began its experience with CAS over 12 years ago. In the beginning, balloon-only angioplasty was employed and, after that, the first phase of stenting was undertaken with balloon-expandable stents. Then, EPDs entered the arena predominated by distal occlusion balloons. During this phase, patients were carefully selected and the procedure evolved with gathering of experience. As the learning curves were optimised, and the results of continuous evaluation26 supported the conviction of offering CAS as an alternative less invasive and effective revascularisation procedure; the practice advanced to the near universal «proceed to endovascular intervention» following conventional diagnostic angiography confirming duplex ultrasound findings. It was during this second phase that dedicated tools such as guiding catheters and sheaths, distal filter and proximal occlusion EPDs, and self-expanding stents came to the fore.
The multi-factorial CAS strategy evolved to a «tailored-approach» in the application of endovascular therapy to a specific patient with a specific lesion and vascular anatomy; wherein the flexibility to use the most appropriate devices and techniques to manage each individual patient exists, without limitations of a RCT protocol such as the restricted use of a particular EPD and stent system. This approach requires an in-depth knowledge of neuro assessment, carotid plaque characteristics, vascular anatomy and technical features of endovascular materials in order to best select the appropriate tools and interventional techniques. Also required is the experience and discipline to implement the «tailored-approach» in a meticulous manner to the entire management strategy. An important element of this concept is the recognition of high-risk cases for CAS dependent primarily on the skill of the interventional vascular specialist, a factor that is substantially more relevant in the field of CAS than other areas of percutaneous interventions. The unit’s «tailored-approach» philosophy to CAS and the technical intervention details have been published previously.23,24
Study design
For relevance to current state-of-the-art interventional practice, we selected from the prospective CAS-registry (that began in July 1997 up to September 2007) all consecutive procedures with intention-to-treat with self-expanding stents (or provisional stenting for restenotic lesions) under neuroprotection with a distal filter or a proximal endovascular clamping device. In addition to supra-regional referrals for CAS to the unit, patients come from afar. Patient selection was restricted to the regional and supra-regional districts, which represent 90 to 95% of lab activity, in order to optimise long-term follow-up data collection. We identified 1,380 patients undergoing 1,523 such procedures (143 patients had bilateral CAS) spanning the period April 1999 to September 2007. The first 119 patients who underwent CAS from July 1997 to April 1999 have been excluded from the present study because the procedures were conducted without neuroprotection as no EPDs were available during this period.
The standard operating procedure is to offer CAS as the preferred strategy for patients requiring revascularisation for severe carotid disease after a multidisciplinary evaluation involving the referral neurologist, the local surgical team and the interventional team. This is based on informed consent and patient choice. Effective clinical governance was instituted including a prospective registry as a measure of quality assurance. This approach is conducted as part of an Institutional Ethics Review Board approved protocol with dispassionate oversight to ensure scientific validation; and the registry has approval of the Italian Society of Invasive Cardiology and contributes data to the Italian Registry for Carotid Stenting.27 All patients had written informed consent for the intervention and a neurological examination by an independent team, wherein a National Institutes of Health Stroke Scale (NIHSS) was performed before and within 24 hours after the procedure.
Demographic, clinical, perioperative and follow-up data were documented in this registry. Indications for intervention included a symptomatic patient with a related carotid stenosis of ≥50% (that is, a lesion-related neurological event in the preceding six months); or an asymptomatic patient with a stenosis of ≥80%. Exclusion criteria included: total occlusion, floating thrombus, severe renal insufficiency (serum creatinine >3.0 mg/dl) and standard contraindications to antiplatelet therapy.
Follow-up schedule included duplex ultrasonography and neurological examination at 24 hours post procedure and subsequently at one, six and 12 months; and thereafter assessment was guided by clinical status. Patients were instructed to notify the responsible physician if any new neurological symptoms occurred after hospital discharge. In addition, follow-up phone interviews were conducted as part of this study to document long-term outcome and data inserted on-line. This was supervised by an independent research scientist enrolled in a Masters programme; and the Villa Maria Health Sciences Foundation was responsible for the database and statistical analysis. A visiting research fellow, undertaking a Masters programme, independently adjudicated events and conducted data evaluation for sake of transparency and external quality assessment. Follow-up to 30 days was complete and follow-up phone interviews were achieved for 1,264 (91.5%) patients. Asymptomatic patients had a follow-up interval to 107 months (8.9 years) and symptomatic patients to 97 months (8.1 years); with an average length of long-term follow-up for the cohort of 8.7 years (95% confidence interval [CI] 8.6 to 8.8).
Data analysis included all strokes, that are, both ipsilateral and contralateral minor/major/fatal strokes, ischaemic or haemorrhagic in nature; and all-cause deaths. Due to the long follow-up period, the broad inclusion of all strokes was chosen as we believe that there would be a potential of misclassification by patients. The primary endpoint was the cumulative incidence of all strokes and deaths at 30 days; and in the case of multiple complications such as stroke leading to death, the most severe was indexed. The secondary endpoint was a composite of the primary endpoint plus cumulative incidence of all strokes or stroke related deaths up to September 30, 2007. For recurrent events, the endpoint was defined by the first occurrence of the event. Regarding procedural risk predictor analyses, that is, 30-day risk of stoke and death; we took the pragmatically relevant approach of including variables that can easily be identified, defined and duplicated when the patient presents for decision-making concerning medical intervention. These variables included demographic and clinical characteristics, and coexisting polyvasculopathy (Table 1). Factors such as challenging aortic arch and complex lesion morphology, and device characteristics are inherently addressed in the «tailored-approach»; and as this approach is being tested in this study these factors were not addressed in the risk predictor model. In the design, conduct and analysis of this study reference to the STROBE guidelines was undertaken to help strengthen the rigour of this study.28
A transient ischaemic attack (TIA) was defined as an acute neurological deficit, of presumed vascular origin related to a focal hemispheric or retinal event lasting <24 hours. Stroke was defined as a new neurological deficit of presumed vascular origin persisting >24 hours, ischaemic or haemorrhagic in nature; and categorised as a minor stroke if the clinical features either resolved completely within 30 days or persisted >30 days and increased the NIHSS by ≤3, and a major stroke if features persisted >30 days and increased the NIHSS by ≥4. A fatal stroke was defined as death attributed to an ischaemic or haemorrhagic stroke.
Statistical analysis
Continuous variables are expressed as mean±SD and nominal variables as count and percentages. For categorical data, group comparisons were made using chi-square test; and the Fisher exact test was used when the predicted contingency table cell values were <5. All probability values were two-tailed, with a value of P<0.05 considered as statistically significant. Univariate analyses followed by multivariate logistic regression methods were used to determine independent predictors of adverse outcomes at 30 days. Kaplan-Meier survival analyses were generated for overall survival and freedom from all-stroke or stroke-related death; and differences between groups were estimated with the use of the log-rank test. All statistical analyses were performed using SPSS software version 15.0 (SPSS, Chicago, IL, USA).
Results
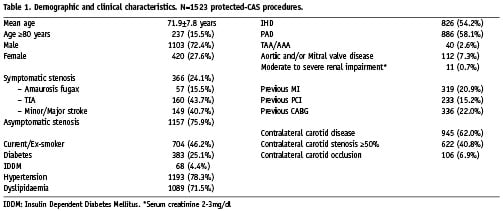
The demographic and clinical characteristics for patients undergoing the specified protected-CAS procedures (N=1523) are listed in Table 1. The mean age was 72 years, with 237 procedures (15.5%) undertaken in octogenarians, defined as age ≥80 years; and 420 procedures (27.6%) in women. This series included 366 symptomatic stenoses (24.1%). Distal filter devices were used in 1,335 procedures (87.6%) and proximal endovascular clamping devices were employed in 188 procedures (12.3%).
Technical success was defined as completion of the index endovascular procedure and immediate morphological success with <30% residual diameter stenosis; along with duplex ultrasound showing absence of pathological acceleration in blood flow (<1.5 m/s). Technical success was achieved in 99.6% of cases and emergency surgery was required in one case due to a trapped filter in the proximal edge of the stent.
Thirty-day outcomes
The 30-day all-stroke/death rate was 1.5% (n=24). This included 11 minor strokes (0.7%), eight major strokes (0.5%) and five deaths (0.3%) giving a 30-day major stroke/death rate of 0.8%. During this period 20 TIAs (1.3%) were documented and there was one in-hospital non-ST elevation myocardial infarction (MI). No stroke or death complications were observed for the following groups: patients aged <60 years (N=111), patients presenting with amaurosis fugax (N=57), patients undergoing CAS for restenosis following previous CEA or CAS (N=100), patients with contralateral carotid occlusion (N=106), and patients with co-existing thoracic and/or abdominal aortic aneurysm (TAA/AAA) (N=40).
Of the five deaths, there were two fatal strokes (an 80 year-old with atrial fibrillation had periprocedural cessation of warfarin and died in-hospital from a cardio-embolic stroke; a 76 year-old had an out-of-hospital seizure and died after admission to a district hospital from a presumed stroke-related death). There were two cardiac-related deaths (an 83 year-old awaiting a staged coronary artery bypass graft [CABG] operation died from an out-of-hospital acute MI; a 63 year-old with heart failure died from out-of-hospital acute pulmonary oedema). The fifth death was in a 77 year-old who died from severe bleeding following a staged CABG during the index admission.
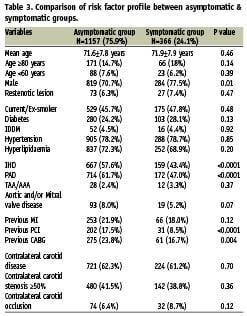
The results of subgroup analyses are listed in Table 2. With reference to symptom status, the 30-day all-stroke/death rates were 1.2% (n=14) for the asymptomatic group, and 2.7% (n=10) for the symptomatic group (odds ratio [OR] 2.3, 95% CI 1.01 to 5.2, P=0.042). The major stroke/death rates were 0.6% for the asymptomatic group and 1.6% for the symptomatic group. The baseline clinical characteristics of patients in both groups were similar (Table 3); with the exception of a significantly higher frequency of ischaemic heart disease (IHD), peripheral arterial disease (PAD), previous percutaneous coronary intervention (PCI) and previous CABG in the asymptomatic group. This may be explained by a selection bias, vis-à-vis an established screening program for carotid disease in patients presenting with disease in the coronary and peripheral districts.
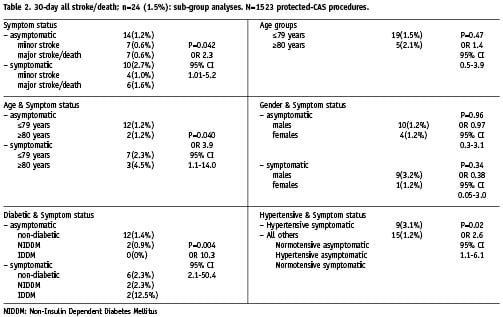
Concerning risk in octogenarians, the 30-day all-stroke/death rates were 1.5% (n=19) for patients ≤79 and 2.1% (n=5) for patients ≥80 years; (P=0.47). Further subset analysis showed that symptomatic octogenarians had a significantly higher risk in comparison with all other groups (OR 3.9, 95% CI 1.06 to 14.0, P=0.040): vis-à-vis asymptomatic group ≤79 years 1.2%, asymptomatic group ≥80 years 1.2%, symptomatic group ≤79 years 2.3% and symptomatic group ≥80 years 4.5%.
In respect of gender, the 30-day all-stroke/death rates for men and women in the asymptomatic group (1.2% vs 1.2%, P=0.96) and symptomatic group (3.2% vs 1.2%, P=0.34) were comparable in both settings.
As regards diabetic status, the subset of symptomatic patients with IDDM had a significantly higher risk in comparison with all other groups (OR 10.3, 95% CI 2.1 to 50.4, P=0.004): asymptomatic non-diabetic patients 1.4%, asymptomatic NIDDM patients 0.9%, asymptomatic IDDM patients (no events), symptomatic non-diabetic patients 2.3%, symptomatic NIDDM patients 2.3%, symptomatic IDDM patients 12.5%.
Regarding hypertensive status, the subset of symptomatic patients with hypertension had a significantly increased risk (3.1%) compared with all other patients (1.2%); (OR 2.6, 95% CI 1.1 to 6.1, P=0.02).
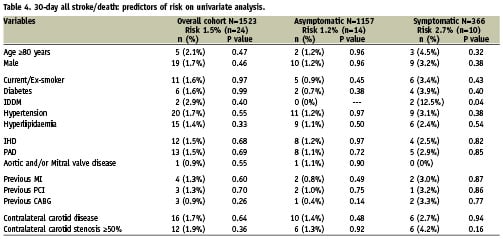
With regard to other potential risk factors, Table 4 lists further univariate analyses undertaken for the overall cohort, and separately for the asymptomatic and symptomatic groups. In a multivariate logistic regression analysis model, symptomatic status was the only independent predictor of the 30-day all-stroke/death rate for the overall cohort (OR 2.3, 95% CI 1.009 to 5.2, P=0.048). With reference to subgroups; in the symptomatic group IDDM was an independent predictor of adverse outcome (OR 6.1, 95% CI 1.2 to 31.5, P=0.03). For the asymptomatic group, there were no independent predictors of 30-day risk of all-stroke/death. Concerning risk in octogenarians, no independent risk factors were identified.
Long-term follow-up
For the overall cohort, during the early and late follow-up periods, there were 166 all-cause deaths including six stroke-related deaths, and 43 non-fatal strokes. Both asymptomatic and symptomatic groups showed no significant difference in survival on Kaplan-Meier analysis (Figure 1A; P=0.09). However, subset analyses based on age demonstrated a significantly higher incidence of all-cause deaths in symptomatic patients ≤79 years compared to asymptomatic patients ≤79 years (Figure 1B; P=0.009). For octogenarians, there was no difference between the groups (Figure 1C; P=0.19).
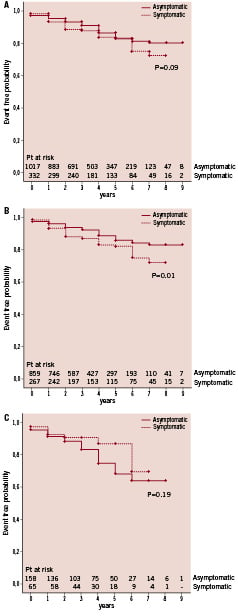
Figure 1. Eight-year Kaplan-Meier survival curves in 1,380 patients undergoing 1,523 tailored protected-CAS procedures. (A) Survival for asymptomatic and symptomatic groups. (B) Freedom from death for patients ≤79 years based on symptom status. (C) Freedom from death for patients ≥80 years based on symptom status.
Kaplan-Meier analyses for the secondary endpoint (composite of primary endpoint plus cumulative incidence of all-strokes or stroke-related deaths at eight years) for both asymptomatic and symptomatic groups are shown in Figure 2A; P=0.003. The cumulative freedom from such events for both the asymptomatic and symptomatic groups were 98% and 93% at one year, 97% and 92% at three years, 96% and 92% at five years, 96% and 92% at eight years. This shows a significantly higher incidence of events for the symptomatic group. Similarly, subset analyses based on age showed that this difference was determined by events in patients ≤79 years (Figure 2B; P=0.003), with no difference amongst octogenarians (Figure 2C; P=0.50).
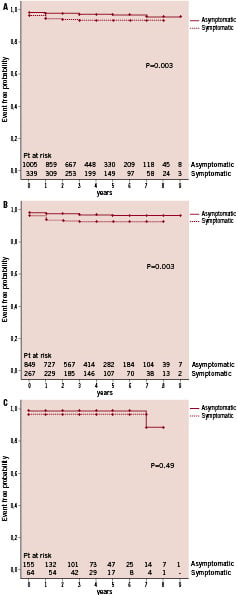
Figure 2. Eight-year Kaplan-Meier cumulative event rates for secondary endpoint: composite of 30-day stroke/death or stroke/stroke-related death beyond 30 days. (A) Analysis of asymptomatic and symptomatic groups. (B) Freedom from secondary endpoint for patients ≤79 years based on symptom status. (C) Freedom from secondary endpoint for patients ≥80 years based on symptom status.
Thus, symptomatic patients ≤79 years have a significantly higher incidence of all-cause deaths and of the specified secondary endpoint compared to asymptomatic patients ≤79 years. The all-cause deaths were not driven by stroke-related deaths, as only three such events were recorded. Concerning octogenarians, events in both asymptomatic and symptomatic groups were determined by death with a very small risk of stroke.
In this series in-stent restenosis ≥50% was detected in 39 cases (2.6%), with reintervention required in 24 circumstances extending a period of four to 48 months from the index procedure; representing a target vessel revascularisation rate of 1.6%. Indications for reintervention were either an in-stent restenotic lesion ≥80% that was asymptomatic in nature or a restenotic lesion ≥50% that was symptomatic in nature.
Discussion
Carotid angioplasty was developed due to a need to provide a less invasive procedure for patients who were deemed too high-risk for open surgical revascularisation. The advancement in endovascular technologies and techniques with dedicated tools has resulted in the evolution of carotid stenting to a refined technique, with a great potential to be applied to routine carotid revascularisation practice.
We believe that on the basis of the present study, carotid stenting with neuroprotection, undertaken with a «tailored-approach» in the hands of well-trained operators29, can be considered to be a safe, efficacious and durable therapy. Firstly, CAS was highly feasible, with minimal exclusion criteria, in a population with extensive coexisting disease. Moreover, we have shown procedural complication rates, in terms of 30-day risk of stroke or death, which are far below the reference bar. In respect of CEA complications, the widely referenced benchmark9 for symptomatic patients is 6% (2.7% in our study) and for asymptomatic patients is 3% (1.2% in our study).
There is much interest and debate concerning risk of CAS in octogenarians with various reports suggesting an increased risk5,6,30 and others suggesting comparable results.31,32 Generally, it is proposed that an increased risk in octogenarians is related to challenging anatomical attributes such as aortic arch elongation, severe calcification, marked tortuosity of vessels, and ulcerated lesions. In the current study, we did not observe a significant increased risk in octogenarians (2.1%). Interestingly, subset analysis did identify a group of patients that do carry the highest risk in our series, which are symptomatic octogenarians (4.5%). On the other hand, the risk for asymptomatic octogenarians was just as low as that for asymptomatic patients ≤79 years (1.2%). It appears that with a «tailored-approach» in the hands of well-trained operators, challenging anatomical characteristics may be successfully managed and, thus, may not be a major determinant of risk in octogenarians. It is reasonable to speculate that other parameters such as impaired intracerebral haemodynamics and cerebral reserve may be more relevant. Nonetheless, it is noteworthy, that the higher risk among symptomatic octogenarians was still very much below the benchmark of 6%.
We wish to emphasise the analysis of outcomes between men and women; as at this time, there is growing appreciation of differences in benefit between the sexes with several therapeutic modalities for cardiovascular disease. Similar disparities have been noted from the RCTs for patients undergoing CEA because of a higher operative risk in women, particularly so for asymptomatic patients wherein women benefited much less than men.33 In the present study, women benefited equally with very low complication rates of 1.2% in both asymptomatic and symptomatic groups.
As regards diabetic status, interestingly, an increased risk was limited to symptomatic patients with IDDM (12.5%). However, caution with interpretation of its clinical significance is required because of statistical limitations due to the small numbers. Similarly, symptomatic patients with hypertension signalled an increased risk (3.1%).
Nonetheless, for the overall cohort, multivariate analysis identified symptom status as the only independent predictor of procedural-risk. Within the symptomatic group, IDDM did prove to be an independent predictor. For the asymptomatic group, there were no identifiable predictors of risk.
So, as a practical guide we are able to say that carotid stenting is a relatively safe procedure, with extremely low risk in asymptomatic patients, both young and old. Though there is a modest increased risk for symptomatic patients (2.7%), the complication rate is still very low with the «tailored-approach», even falling below the referenced benchmark of 3% for asymptomatic patients. However, increased attention to symptomatic patients who are octogenarians, or have IDDM or hypertension is required. It is noteworthy, that in several subsets of patients CAS appears to be particularly safe: age ≤60 years, amaurosis fugax as presenting symptom, restenotic lesions, contralateral carotid occlusion and coexisting aortic aneurysmal disease. Indeed, the «tailored-approach» appears to reduce complications to a new threshold, and continued refinement in techniques and materials will improve patient-outcomes.
Regarding long-term risk, the results are very reassuring with a high freedom from stroke/stroke-related death at eight years suggesting that carotid stenting is efficacious and durable. A notable observation is a consistent modest increased risk of death and neurological events amongst symptomatic patients ≤79 years as compared to asymptomatic patients ≤79 years. One possible explanation may be the systemic and diffuse nature of plaque inflammation and vulnerability; which is supported by a report highlighting a high risk of vascular death in patients with a history of ischaemic stroke.34 With regards to neurological events, it does raise interesting hypotheses, and brings into perspective the importance of coexisting intracranial atherosclerotic disease, which needs to be studied and addressed. Also of note is the low restenosis rate and need for reintervention.
Well-conducted applicable RCTs are essential in establishing equivalence or superiority of new therapeutic interventions. Ongoing large RCTs will hopefully address the uncertainty that exist regarding CAS. Nonetheless, the defining strength of this data reflects treatment for all-comers in a «real world» setting, managed with a «tailored-approach» to intervention and mandatory use of EPDs. This is especially relevant in the carotid district, where we believe the choice of tools is of paramount importance in the safe application of this technique. In the reality, the data echoes the true day-to-day clinical decision making and application of carotid stenting which is influenced by knowledge from experience, RCTs and supplementary well-conducted, comprehensive and prospective registries.
A critical appraisal of the reported data
The main issue raised by a critical evaluation of the reported data is the question pertaining to an explanation for the very good results in our study.
Firstly, evidence is accumulating from registry-data that CAS is producing good results from experienced high-volume centres showing a 30-day stroke/death rate of 2.2%8.
Before discussing the main factors determining a good outcome we will firstly clarify some other points:
A. May our good results be related to patient selection? To further qualify the statement «the defining strength of this data reflects treatment for all-comers» we wish to reinforce that there were minimal exclusion criteria for all-comers and with a «near universal proceed to endovascular intervention following conventional diagnostic angiography confirming duplex ultrasound findings», meaning that more than 99% of patients who required revascularisation proceeded to the endovascular approach. Hence patient selection bias was not a factor in our population.
B. May our good results be related to delay in treatment? We do not have data in our registry pertaining to time from qualifying event in our symptomatic group of patients to intervention. For patients presenting with a stroke, our procedural protocol is to delay intervention for two to four weeks. Hence, delay in the referral pathway from referring hospitals is not a significant factor. In the EVA-3S study12 79.7% of patients were treated beyond two weeks with up to 54.4% treated beyond four weeks. In the ICSS study35 73% of patients were treated beyond two weeks.
Apart the previous considerations, we wish to emphasise that our study suggest very good results can be obtained by the following three factors: i/ mandatory neuroprotection, ii/ a tailored-approach and iii/ a high-level of experience.
i. Mandatory neuroprotection. Certainly, there is no randomised trial to assess the efficacy of neuroprotection and there is unlikely to ever be one. SPACE, EVA-3S (initially) & ICSS12,13,35 did not mandate neuroprotection. Post hoc analysis comparing outcomes in patients treated with and without EPD is fundamentally flawed. According to a subanalysis of the SPACE trial36 the 30-day rates of ipsilateral stroke or death were similar (p=0.40): 8.3% in those treated with EPDs (n=145) and 6.5% in those treated without EPDs. From this the authors conclude that EPDs are not necessary. It is reasonable to suggest that physicians did not use EPDs in low-risk cases, reserving the use of EPDs in high-risk cases. But, the results achieved in the presumed «low-risk» cases treated without EPDs were just as high in the presumed «high-risk» cases treated with EPDs. It is not beyond reasonable thinking, that it is possible, if EPDs were used in the presumed «low-risk» cases the risk may have been substantially lower, and since this represented the great majority of patients the overall results may have been much better. For this reason, such post hoc analysis is scientifically flawed.
In our practice, neuroprotection is mandatory and such an approach standardises our procedure which contributes to the effectiveness. Experience is thus maintained at a high-level in the use of such devices. In addition, this approach allows experience to be gained in the use of multiple devices which are readily available on the shelf, allowing the safe application of individual treatment.
ii. Tailored-approach. We strongly believe that the tailored-approach is especially relevant to the good outcomes. Many units use one access system, one type of EPD and one stent type. Currently, there is much debate about the relevance of closed versus open cell stent designs. In our practice we tailor the choice of stent. Again, there is no randomised trial assessing these issues. We believe that experience with a wide range of devices allows the operator to choose from the shelf the most appropriate tools and techniques for the safe application of CAS for the individual patient.
iii. High-level of experience. “An important element of this concept is the recognition of high-risk cases for CAS dependent primarily on the skill of the interventional vascular specialist...” “These results underscore that with experienced operators – built on a philosophy of dedicated training...” Obviously, experience is difficult to quantify and is related to number of procedures, quality of experience and the background skill of the interventionalists. The CAS First Consensus Document of the ICCS-SPREAD Joint Committee29 addressed credentials and competency. The minimum recommended training to achieve competence is as follows:
1. At least 150 procedures of supra-aortic vessel engagement (during diagnostic as well as interventional procedures) within two years, of which at least 100 as the primary operator;
2. At least 75 carotid stenting procedures, of which at least 50 as the primary operator, within a 2-year fellowship.
The minimum requirement to maintain technical skill is 50 CAS procedures as primary operator per year. The learning curve flattens after about 80 interventions with regard to procedural risk and after about 160 interventions with regard to procedural time. In addition, an environment of learning/training significantly facilitates performance, not only for the operator but the entire catherisation lab staff and post-op care staff. Also important, independent of numbers, is the length of time involved in the delivery of CAS as an option and the time and discipline it takes to truly appreciate and deliver the tailored-approach in a meticulous manner to the entire management strategy. This is all part of the experience we represent.
The position of the consensus panel is based on the assumption that CAS must be performed only by high-level and well-trained interventionalists. Certainly, we endorse this position.
We believe that the operator experience needed, in the planning of EVA-3S & SPACE trials12,13, were clearly underestimated. In the EVA-3S trial the total experience of the interventionalists required at least 12 CAS or at least 35 supra-aortic trunk interventions of which at least five were in the carotid artery. A variation was made wherein an interventionalist who did not fulfil the requirement was allowed to enrol patients and perform the procedures under supervision until the predefined criteria was attained. Indeed, 85% of interventionalists in this trial had ≤50 cases in total. In the SPACE trial, interventionalists had to show proof of at least 25 successful consecutive percutaneous transluminal angioplasty or stent procedures.
Clearly, these trials were not designed to assess the effect of experience. Again, post hoc analyses comparing outcomes in patients treated by inexperienced and experienced physicians are flawed based on the arguments discussed above regarding neuroprotection. Additionally, the definition of experience is not consistent with our view.
Regarding the ICSS trial35, an expert interventionalist was defined by having performed at least 50 stenting procedures anywhere, of which at least 10 should be in the carotid artery. Centres with no or little experience could join the trial as a probationary centre «supervised centre» wherein patients were enrolled and treated under the supervision of a proctor. Subsequently, after the centre achieved the minimum requirement of 10 CAS it was promoted to an experienced centre.
Study limitations
Comparison of the results of this study with RCTs is limited by differences in time periods, case mix, completeness of neurological evaluation, and non-standardised endpoints. In addition, due to the long follow-up period and use of phone interviews, there would be a potential of misclassification of events. For these reasons, the broad inclusion of all strokes was chosen in order to be all inclusive. The «tailored-approach» is not a fixed protocol but a continuously evolving therapy, and though this may appear as a limitation, it is exactly this concept we are testing as new technology comes on stream. Long-term follow-up has been completed in 1,264 (91.5%) patients. A drop-out of 8.5%, inherent to this long-term study model, has to be considered an under-powering factor in the comparison between CAS and CEA cumulative complications and survival-free rates.
Conclusions
Carotid artery angioplasty and stenting has assumed a defining role in the optimal management of patients with carotid bifurcation disease. The results from this large cohort demonstrate that carotid stenting for all-comers in a «real-world» setting is safe and efficacious, and durable in the long-term prevention of stroke. These results underscore that with experienced operators – built on a philosophy of dedicated training, a «tailored-approach», and mandatory neuroprotection – carotid stenting is an effective procedure. Emerging technologies and further innovations applied in this way, to continuously refine the technique and improve on the management of patient care, may move us that much closer to stroke prevention.
Acknowledgements
A special thanks to Romina Giulianini and Luri Turchi for the accurate database data entry and management.

