Abstract
Aims: To investigate long-term outcomes achieved in high-surgical-risk patients and other clinically-relevant subgroups after carotid stenting with the NexStent and Filterwire EX/EZ devices.
Methods and results: CABERNET, a prospective, multicentre, single-arm trial, enrolled 454 patients with extracranial internal carotid artery stenosis (symptomatic ≥50%, n=110; asymptomatic ≥60%, n=344). Early outcomes at one year have been reported. The 3-year Kaplan-Meier estimated event rates were: 7.2%, all stroke; 2.8%, major stroke; 4.8%, ipsilateral stroke; 17.7%, all death; 7.1%, myocardial infarction; 4.4%, target vessel revascularisation. Asymptomatic patients had significantly fewer major strokes than symptomatic patients (1.9% vs. 5.7%, P=0.03) and patients <80 years had significantly fewer ipsilateral strokes than those ≥80 years (3.2% vs. 10.7%, P=0.002). Stroke outcomes did not differ significantly between patients with anatomical risk factors compared with those with comorbid medical risk factors.
Conclusions: Long-term outcomes achieved in high-surgical-risk patients with the NexStent and Filterwire EX/EZ devices are favourable. Outcomes may be better in asymptomatic patients or those younger than 80 years of age. These data will be helpful in estimating short-term risks of carotid stenting and balancing these risks against the long-term benefit of stroke prevention.
Introduction
Stroke is a major cause of mortality and morbidity throughout the world, and the medical, social, and economic consequences of stroke are likely to increase given an ageing population1,2. Large, prospective, multicentre, randomised controlled trials have shown that carotid endarterectomy (CEA) is superior to medical treatment for stroke prevention in both symptomatic3-6 and asymptomatic7,8 patients with significant extracranial internal carotid artery disease. The strength of this evidence has helped to establish CEA as the accepted gold standard for treating extracranial internal carotid artery disease1,9-11.
The desire for less invasive therapies for extracranial internal carotid artery disease has motivated the development of carotid artery stenting (CAS) with distal embolic protection as a potential alternative therapy. As patients at the highest risk of adverse events from CEA may benefit most from CAS1,12,13, CAS has been studied most widely in high-surgical-risk patients14-19, for whom the short-term perioperative risk of CEA might otherwise outweigh its potential long-term benefit of stroke prevention1,12,13.
Despite several randomised trials and a number of prospective non-randomised studies, there is still considerable debate about the relative safety and efficacy of CAS compared with CEA in a broader patient population. The main goal of both CEA and CAS is the prevention of stroke in the years following revascularisation and comparison of the two treatment modalities depends critically on the rates of stroke observed during long-term follow-up (not just the periprocedural period). The possibility that some patients are better suited to either procedure has also heightened interest in subgroup analyses of clinical trial data.
Data on event rates beyond one year after surgery or CAS are very limited. The SAPPHIRE trial is the only major randomised trial comparing CEA and CAS in high-surgical-risk patients, and this study showed no difference in the rate of stroke or other major adverse events at one16 and three years14. Subgroup analyses, though of great interest to the medical community, were limited by the relatively small sample size of the randomised population of the SAPPHIRE study14. Long-term outcomes have been reported for two other randomised controlled trials comparing CEA and CAS, although these trials were not confined to high-surgical-risk patients. In the SPACE trial, there were no significant differences between CEA and CAS in the rate of stroke or death at two years20. In the EVA-3S trial, the risk of stroke or death at four years was higher with CAS compared with CEA21. These differences were driven by an excess of events during the periprocedural period, with no significant differences between CEA and CAS in the rate of stroke or death from day 31 to four years.
We now report the 3-year results from the CABERNET study, a large, prospective, multicentre, single-arm CAS trial designed to evaluate the safety and efficacy of the NexStent used in combination with the Filterwire EX/EZ distal protection system in high-surgical-risk patients. We include results from subgroup analyses that explored the potential influence of symptom status, risk factor profile, and age on clinical outcomes. The 30-day and 1-year results from this trial have been reported15.
Methods
Study design, population and methods
The CABERNET study design, subject inclusion criteria, and methods have been described in detail15, but will be briefly summarised here.
The study was approved for investigational device exemption by the U.S. Food and Drug Administration, conducted in accordance with the Declaration of Helsinki, and registered on the National Institutes of Health clinical trial registry (ClinicalTrials.gov identifier: NCT00600327). The study protocol was approved by the Institutional Review Boards of the 19 participating study centres (15 in the USA, 3 in Germany, and 1 in Argentina). Before participating in the study, all study investigators were required to have performed at least 20 carotid stenting procedures with distal embolic protection. In addition, each centre conducted a training session for investigators on the combined use of the NexStent and FilterWire devices before the study began.
All patients provided voluntary written informed consent before participating in the study. Patients were enrolled between 20 February 2002 and 10 March 2004, and the 3-year follow-up period was completed on 6 June 2007. Patients with internal carotid artery stenosis (assessed by angiography: ≥50% for symptomatic and ≥60% for asymptomatic patients), who were considered to have a high risk of complications from CEA, were eligible to participate. To be included, patients needed to meet at least one anatomical or at least one Class I comorbid criterion, or at least two Class II comorbid criteria (Table 1).
Carotid duplex ultrasonography was performed within 30 days before the procedure. Patients received the following antiplatelet therapy before CAS: aspirin (325 mg daily, at least 72 h before) and either clopidogrel (75 mg daily, 3 days before; or 150 mg daily, 2 days before; or 450 mg, at least 4 h before) or ticlopidine (250 mg twice daily, 2 days before). Neurological assessments (National Institutes of Health Stroke Scale [NIHSS], Barthel Index, and Modified Rankin Scale) were performed by NIHSS-certified neurologists within 24 hours before CAS.
Study devices and procedure
The CAS procedure, which has been described in detail15, was performed using a NexStent™ Carotid Stent and Delivery System (Boston Scientific Corporation, Natick, MA, USA) and a FilterWire EX®/EZ™ Embolic Protection System (Boston Scientific Corporation, Natick, MA, USA). Following the stent procedure, patients were instructed to take aspirin (325 mg daily, indefinitely), and either clopidogrel (75 mg daily, for a minimum of 30 days) or ticlopidine (250 mg twice daily, for a minimum of 30 days). Follow-up visits were scheduled for 30 days, six months, and one, two, and three years after the CAS procedure. At each follow-up visit, a standard cardiopulmonary and neurological history was taken, a physical examination was performed and NIHSS-certified neurologists conducted neurological assessments.
Clinical outcomes
During the 3-year period after CAS, the main clinical outcomes of interest were the overall rates of stroke (all; major; ipsilateral), death, myocardial infarction (MI), and the need for target vessel revascularisation (TVR). Stent patency was assessed by measuring maximum peak systolic velocity and end-diastolic velocity by duplex ultrasonography.
In addition to the clinical outcomes, all patients were assessed for the relative rates of stroke, death, MI, and TVR for clinically-relevant subgroups of patients. These subgroups were defined relative to: (i) symptomatic status (asymptomatic vs. symptomatic); (ii) type of risk factor (anatomic vs. comorbid); and (iii) age (<80 years vs. ≥80 years of age).
Statistical analyses
Data were analysed on an intention-to-treat basis. The estimated rates of all stroke (comprising major, minor, ipsilateral, and contralateral stroke events), major stroke, ipsilateral stroke, all death, MI, and TVR in all patients during the 3-year period were determined by Kaplan-Meier (K-M) analyses. Post hoc analyses were conducted to estimate the rates of the main clinical outcomes of interest in the subgroups (asymptomatic vs. symptomatic; anatomic vs. comorbid medical risk factors; <80 years vs. ≥80 years of age). Patients who had both anatomical and comorbid medical risk factors were included in the comorbid subgroup for analysis. Differences between the subgroups were compared using a log-rank test; P values <0.05 were considered statistically significant. Statistical analyses were performed using the SAS software, Version 8 or later (SAS Institute Inc., Cary, NC, USA).
Results
Demographics and subject disposition
The demographics and baseline clinical characteristics of the patients enrolled in this study have been described in detail15. In brief, the mean age (± standard deviation) of patients was 72.5±8.6 years, 65.4% (n=297/454) were men, and 33.0% (n=150/454) had diabetes mellitus. In terms of the subgroups, 75.8% (n=344/454) of patients were asymptomatic, 63.4% (n=288/454) had anatomical risk factors, and 77.8% (n=353/454) were <80 years of age.
At the time of the CAS procedure, the mean lesion length was 15.0±6.8 mm, the mean reference vessel diameter was 5.61±1.0 mm, and the mean extent of stenosis was 83.7±9.8%. The stent was successfully implanted in 98% (n=443/454) of patients.
During the 3-year study period, the follow-up rate was 95.9% (n=398/415) at one year, 86.2% (n=343/398) at two years, and 83.7% (n=309/369) at three years.
Clinical outcomes: all patients
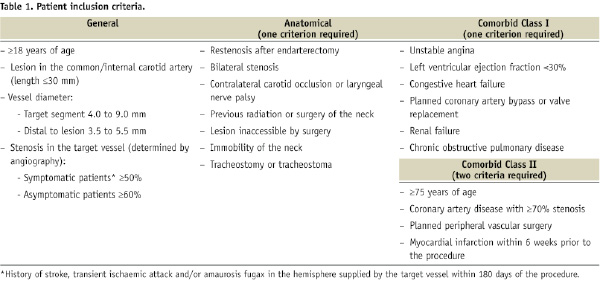
The K-M estimate of all stroke events was 3.5% at 30 days and, thereafter, increased in a relatively stable linear fashion to 7.2% at three years (Figure 1A). The annualised hazard rate for all stroke from 30 days to three years (AHR 30 days to three years) was 1.3% per year. A similar pattern was observed for major stroke and ipsilateral stroke, with approximately half of all events occurring in the periprocedural period (Figure 1A). The K-M estimate for major stroke was 1.3% at 30 days and 2.8% at three years (AHR 30 days to three years=0.5% per year), and for ipsilateral stroke was 2.9% at 30 days and 4.8% at three years (AHR 30 days to three years=0.7% per year; Figure 1A). The K-M estimate for contralateral stroke was 0.7% at 30 days and 2.6% at three years (AHR 30 days to three years =0.7% per year), thus approximating the annualised risk of ipsilateral stroke in the stented vessel after 30 days. The K-M estimate of all death increased steadily during the follow-up period to 17.7% at three years (AHR 30 days to three years=5.9% per year; Figure 1B), reflecting the high-surgical-risk patient population. Few deaths, however, were from neurological or cerebrovascular events (1.3% at three years); the major causes of death were cardiac-related events (31.5% of all deaths; n=23/73) and carcinoma (15.1% of all deaths; n=11/73). The K-M estimate of MI was 0.4% at 30 days during the periprocedural period and increased to 7.1% at three years during the follow-up period (AHR 30 days to three years=2.3% per year; Figure 1C).
The mean maximum peak systolic velocity within the internal carotid artery remained low during the follow-up period, indicating continued stent patency and minimal restenosis (Figure 2). As duplex ultrasound was optional at the 2- and 3-year study visits, the number of patients at these time points was lower than that at the 1-year study visit. However, in support of the findings of low mean maximum peak systolic velocity, the estimated rates of TVR were low and constant during the study; the 3-year K-M estimate was 4.4% (AHR 30 days to three years=1.5% per year; Figure 1D).
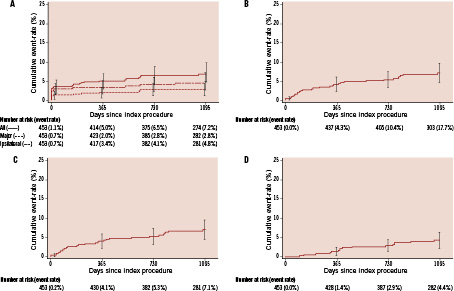
Figure 1. Kaplan-Meier curves depicting the 3-year cumulative event rates of stroke (all stroke [major, minor, contralateral and ipsilateral stroke], solid line; major stroke, dotted line; and ipsilateral stroke, dashed line) (panel A), all death (panel B), myocardial infarction (panel C), and target vessel revascularisation (panel D). Error bars represent the standard error of the event. The number of patients who were event-free and still at risk during each year and the Kaplan-Meier estimate for each year are presented below each graph.
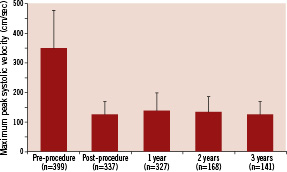
Figure 2. The mean (+ standard deviation) maximum peak systolic velocity within the internal carotid artery before and during the study. As duplex ultrasound was optional at the 2- and 3-year study visits, the number of patients at these time points was lower than that at the 1-year study visit.
Clinical outcomes: subgroups
The 3-year K-M estimate of major stroke was significantly lower in asymptomatic patients than in symptomatic patients (1.9% vs. 5.7%, log-rank P=0.03; Table 2). The K-M estimates of all stroke (asymptomatic 6.2% vs. symptomatic 10.5%, P=0.09), ipsilateral stroke (4.0% vs. 7.6%, P=0.09; Figure 3A), and neurological death (0.7% vs. 3.1%, P=0.06) also trended lower in asymptomatic patients, although these differences did not achieve statistical significance (Table 2). Rates of all death, non-neurological death, MI, and TVR were similar between asymptomatic and symptomatic patients (Table 2).
Figure 3. Kaplan-Meier curves depicting the 3-year cumulative event rates of ipsilateral stroke for symptomatic (solid line) or asymptomatic (dotted line) patients (panel A), for patients who had anatomical risk factors (solid line) or comorbid medical risk factors (dotted line) (panel B), and for patients who were <80 years old (solid line) or ≥80 years old (dotted line) (panel C). Error bars represent the standard error of the event. P values were calculated using the log-rank test and are presented for the difference in event rates through three years. The number of patients who were event-free and still at risk during each year and the Kaplan-Meier estimate for each year are presented below each graph.
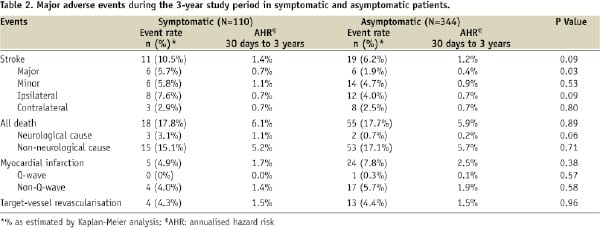
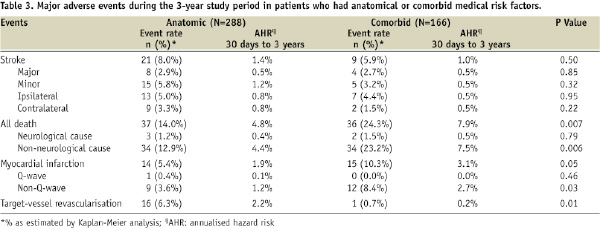
The 3-year K-M estimate of stroke did not differ significantly between patients who were deemed high risk because of anatomical versus comorbid medical risk factors (Table 3; Figure 3B). However, the 3-year K-M estimate of all death was significantly lower for patients with anatomical risk factors, compared with patients with comorbid medical risk factors (14.0% vs. 24.3%, P=0.01; Table 3). This difference in the all death rate was driven by a difference in the rate of non-neurological death (Table 3). Patients with comorbid medical risk factors also had a higher rate of MI (comorbid 10.3% vs. anatomical 5.4%, P=0.05), but had a lower rate of TVR (0.7% vs. 6.3%, P=0.01; Table 3).
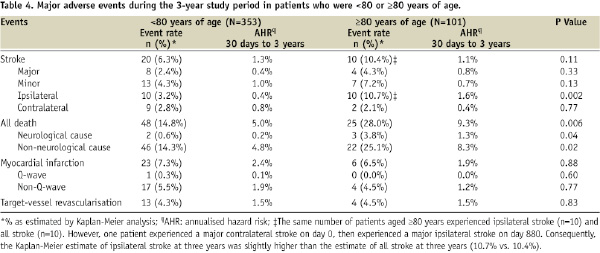
The 3-year K-M estimate of ipsilateral stroke was significantly lower in patients <80 years of age than in patients ≥80 years of age (3.2% vs. 10.7%, P=0.002; Figure 3C). The 3-year K-M estimate of all death was also significantly lower in patients <80 years of age than in patients ≥80 years of age (14.8% vs. 28.0%, P=0.006; Table 4). The 3-year K-M estimates of MI and TVR were similar in the two age groups (Table 4).
Discussion
Although carotid revascularisation is associated with a higher risk of periprocedural events than medical therapy, in exchange it is expected to decrease the long-term risk of stroke. The overall evaluation of carotid revascularisation, therefore, must balance any short-term risks of periprocedural events with the anticipated long-term benefits of a decreased risk of stroke over time. We have published data regarding the incidence of periprocedural and 1-year events after CAS with embolic protection5, and now report the long-term follow-up data from CABERNET, a large, prospective, multicentre, single-arm CAS trial. These results indicate a relatively low incidence of ipsilateral stroke from 30 days to three years after CAS (0.7% per year). Notably, this incidence is very similar to the incidence of stroke in the contralateral carotid artery (also 0.7% per year). Based on post hoc subgroup analyses, our results further suggest that the annualised risk of stroke may be lower in asymptomatic patients and patients younger than 80 years of age. Our data on the long-term outcomes of CAS may help clinicians estimate the short- and long-term risks after CAS with the known risks of CEA, and balance the potential long-term benefits of a decreased risk of stroke over time with either form of revascularisation, compared with medical therapy alone.
There are few published reports from prospective studies on the long-term stroke outcomes after CAS with embolic protection in high-surgical-risk patients. The results from the SAPPHIRE trial are of particular interest as this randomised trial compared CAS with CEA in high-surgical-risk patients and long-term follow-up data from this trial have been reported14. Notably, although our study included almost three times as many CAS patients as those in the SAPPHIRE trial (n=454 vs. n=167)14, the 3-year stroke outcomes in our study (e.g., all stroke 7.2%) were lower than those reported for either the CAS (all stroke 10.1%) or the CEA (all stroke 10.7%) arms of the SAPPHIRE trial14. In terms of other clinically important outcomes, the 3-year rates for all death, MI, and TVR in CABERNET were within the expected ranges for this high-risk population (i.e., given the influence of generalised atherosclerosis on the rates of death and MI), and were also similar to the rates reported from the SAPPHIRE trial14.
Consistent with the results from SAPPHIRE, the highest incidence of stroke events in CABERNET occurred during the immediate (30-day) postprocedural period. Our finding of a low and stable long-term stroke rate with CAS thereafter is indirectly supported by the long-term results from large randomised controlled trials that have compared CAS with CEA in conventional-risk patients. The 2-year results from the SPACE trial20 and the 4-year results from the EVA-3S trial21 indicate that there are no significant differences in stroke outcomes between CAS and CEA beyond the periprocedural period; the long-term stroke rates for both CAS and CEA were relatively low and stable. Further refinement of our understanding of the expected long-term stroke outcomes after CAS with an embolic protection device, particularly for high-surgical-risk patients, should be possible when the long-term results from a number of ongoing carotid registry studies are published9,22. The low ongoing annualised risk of ipsilateral stroke (AHR 30 days to three years =0.7% per year) and contralateral stroke (AHR 30 days to three years =0.7% per year) suggest that the important improvements will be made in reducing adverse outcomes within the first 30 days after the procedure.
As anticipated, baseline symptom status appears to influence the long-term stroke outcomes after CAS. Consistent with the CABERNET results at 30 days and one year15, we found that the estimated rate of major stroke at three years was still significantly lower in asymptomatic patients, compared with symptomatic patients. A more favourable outcome after CAS for asymptomatic patients has been a consistent finding in short-term studies of CAS9. Long-term outcomes in CABERNET also compare favourably with the long-term stroke outcomes from a number of large, randomised controlled trials that studied the effects of CAS, CEA, or medical management on conventional-risk symptomatic patients. For example, the 3-year estimated rate of ipsilateral stroke in symptomatic CABERNET patients (7.6%) is similar to, or lower than, the long-term ipsilateral stroke rates in the CAS and CEA arms of the SPACE20 or the EVA-3S21 trials (9.5% with CAS and 8.8% with CEA at two years in SPACE and 11.1% with CAS and 6.2% with CEA at four years in EVA-3S). The rate of ipsilateral stroke in symptomatic patients in CABERNET is also substantially lower than the rates seen with medical management in similar patients (e.g., symptomatic with moderate-to-severe stenosis) in the North American Symptomatic Carotid Endarterectomy Trial6 (26.0% at two years) or the European Carotid Surgery Trial5 (21.4% at approximately six years) trials. Long-term results for asymptomatic patients in CABERNET (3-year ipsilateral stroke rate 4.0%) are also consistent with results from the randomised trials, such as Asymptomatic Carotid Artery Study (ipsilateral stroke through five years plus perioperative stroke and death was 5.1% in the CEA arm and 11.0% in the medical therapy arm)7 and Asymptomatic Carotid Surgery Trial (5-year stroke rate was 6.4% in the CEA arm and 11.8% in the medical therapy arm)23. These randomised trials studied conventional risk, asymptomatic patients, as opposed to the high-surgical-risk patients studied in CABERNET. Although we recognise the challenges of making indirect comparisons with previous studies, our results suggest that in high-surgical-risk patients, long-term CAS outcomes may be similar to those of CEA, and potentially better than the outcomes with ongoing medical management.
We also anticipated that age might influence long-term stroke outcomes after CAS. Similar to our finding of an age-related effect on the rate of ipsilateral stroke at one year15, we found that the estimated rate of ipsilateral stroke at three years was lower in patients aged younger than 80 years. Whether the age-related results apparent in our study are due to age per se, or whether the effect of advanced age is confounded by other factors that might affect CAS outcomes24, is unclear. This is clinically relevant because in some centres, at least 25% of patients requiring carotid revascularisation are older than 80 years of age25. Careful selection of patients, however, may allow experienced operators to perform CAS successfully in patients older than 80 years of age24,26.
Contrary to what might have been anticipated, we did not detect worse stroke outcomes in patients with comorbid medical risk factors, compared with patients who had anatomical risk factors. Although the rates of all death and MI were significantly higher in the comorbid group, the annualised rate of stroke did not differ significantly between the two groups.
This study had several limitations. As we did not include either medical management or CEA in our single-arm study design, we do not know exactly how our long-term results with CAS would have compared with results obtained with other treatment options. In terms of generalisability, our results in high-surgical-risk patients may not be applicable to patients who have a low or moderate risk of complications from CEA. Furthermore, we realise that treatment with NexStent and/or Filterwire is not suitable for all lesion types. Patients with lesions incompatible with use of the stent system or distal protection device were excluded from the CABERNET study, and data are not available regarding the number of patients who were screened, but not enrolled, for this reason. Another limitation is that our sub-group analyses were not pre-specified in the original protocol and should, therefore, be viewed as hypothesis-generating rather than conclusive analyses. We also recognise that extrapolating the long-term results achieved with the NexStent and Filterwire EX/EZ devices to other CAS and distal embolic protection devices should be done with caution. Finally, we note that the version of the NexStent that we studied was removed from the market, subsequent to completion of enrolment in CABERNET, because of problems with the delivery catheter. Nevertheless, these results can help us understand the long-term clinical experience achieved with carotid stenting as well as help define expectations with future generations of the NexStent (e.g., the Adapt™ Carotid Stent).
In conclusion, we have shown that the early favourable clinical outcomes achieved with the NexStent and Filterwire EX/EZ embolic protection device in high-surgical-risk patients are durable out to three years of follow-up. The rates of stroke events after the periprocedural period were low and relatively stable. Post hoc analyses suggested that the rate of major stroke may be lower in asymptomatic patients and the rate of ipsilateral stroke may be lower in patients younger than 80 years of age. The results from this large, prospective, multicentre trial will help define the long-term outcomes that clinicians can expect when employing CAS with an embolic protection device in high-surgical-risk patients. In particular, our data should help clinicians balance the short-term periprocedural risks of CAS with the potential long-term benefits of a decreased risk of subsequent stroke.
Acknowledgements
This study was sponsored by Boston Scientific Corporation, Natick, MA. In compliance with the Uniform Requirements for Manuscripts, established by the International Committee of Medical Journal Editors, the sponsor of this study did not impose any impediment, directly or indirectly, on the publication of the study’s results. The authors acknowledge the helpful comments from Ruth Starzyk and Olga Tsukurov from Boston Scientific, and the statistical input from the biostatisticians at Boston Scientific. The authors also acknowledge the independent medical writing assistance provided by ProScribe Medical Communications (www.proscribe.com.au), funded by Boston Scientific Corporation. ProScribe’s services complied with international guidelines for Good Publication Practice.
Appendix
Participating institutions (study sites):
This multicentre research study was conducted at 19 sites. The participating institutions included SUNY-Millard Gates Hospital, Buffalo, NY, USA; Hoag Memorial Hospital, Newport Beach, CA, USA; Siegburg Heart Center, Siegburg, Germany; The Sanger Clinic, Charlotte, NC, USA; Leipzig Heart Center, Leipzig, Germany; Albany Medical Center, Albany, NY, USA; Columbia/NY Presbyterian, New York, NY, USA; Union Memorial, Baltimore, MD, USA; Harrisburg Hospital, Harrisburg, PA, USA; St. Francis/Allegheny General Hospital, Pittsburgh, PA, USA; Swedish Medical Center, Seattle, WA, USA; St. Luke’s/St. Joseph’s Hospital, Phoenix, AZ, USA; Lenox Hill Hospital, New York, NY, USA; Ochsner Clinic, New Orleans, LA, USA; El Camino Hospital, Mountain View, CA, USA; Favaloro Institute, Buenos Aires, Argentina; Bethanian/St. Katarinen, Frankfurt, Germany; UPMC-Shadyside Hospital, Pittsburgh, PA, USA; Cleveland Clinic Foundation, Cleveland, OH, USA.

