
Introduction
Large clinical series of carotid artery stenting (CAS) using conventional carotid stents have shown that 40-80% of adverse neurologic events within the first 30 days occur post-procedurally rather than intraprocedurally, and often several days after the procedure1. Indeed, first-generation carotid stent use is associated with post-procedural cerebral embolism2 that largely results from intraluminal plaque prolapse through the struts3, a phenomenon which is not eliminated by the classic closed-cell design4.
Sequential cerebral imaging with diffusion-weighted magnetic resonance (including routine imaging at baseline, 24-48 hours and 30 days5) demonstrated that the MicroNET-covered embolic prevention stent CGuard™ (InspireMD, Tel Aviv, Israel)5,6,7,8,9 minimises intraprocedural and eliminates post-procedural cerebral embolism5,6,7,8,10, providing a basis for larger-scale clinical endpoint-oriented studies11,12. The device, combined with a tailored use of intraprocedural embolic protection (the MicroNET embolic prevention occurs only after stent deployment), demonstrated favourable short-term clinical outcomes (30-day death/stroke/MI rate <1%)5,7,10, but its longer-term safety and efficacy need to be established.
Methods
PARADIGM10 is a non-industry-funded, prospective academic study in all-referrals-tracked symptomatic and asymptomatic carotid stenosis with a multi-specialty NeuroVascular Team (NVT; angiologist/cardiologist, vascular surgeon, neurologist)10 making decisions on revascularisation including establishment of indication and endovascular approach feasibility assessment. The study enrolled unselected, consecutive patients (flow chart in reference 10) with an independent neurologist evaluation at baseline, periprocedurally and at one and 12 months, and with events adjudication by an independent clinical events committee (CEC)10. Details regarding methodology are provided in Supplementary Appendix 1 and in reference 10.
Results
Over 12 months, 108 consecutive patients with NVT-indicated carotid revascularisation were enrolled10. The endovascular route of revascularisation was considered feasible in 101 subjects (70% men, 55% symptomatic including symptoms within the preceding 14 days in 22% of the study cohort and 9 strokes-in-evolution); 7/108 patients were referred for carotid endarterectomy (CEA)10. In the CAS cohort (106 arteries), 25 lesions (23.6%) were thrombotic on both duplex ultrasound (DUS) and angiography. With all procedures neuroprotected, proximal (flow reversal) device use was overall nearly 50%, with a higher prevalence in symptomatic lesions10. Stents were routinely coronary-like optimised, using large-diameter balloons10 and higher pressures10 than conventional CAS1, leading to single-digit residual diameter stenosis10. Heparinisation was consistent with the device instructions for use (activated clotting time throughout the procedure of >250 s)10. Study device use was 100% (zero other stent type[s] CAS)10 throughout the study period.
CLINICAL EVENTS BY 12 MONTHS
By 30 days there was one (0.9%) adverse event – periprocedural, clinically asymptomatic extension of a prior cerebral infarct zone in a hypotensive patient (CEC-adjudicated as minor stroke)10. No death, large stroke or myocardial infarction occurred by 30 days10. By 12 months, there were no patient withdrawals, and no patient was lost to follow-up. Between 30 days and 12 months, there were no strokes or stroke-related deaths but 4 non-stroke deaths occurred, including 1 cardiac death (heart failure exacerbation) and 3 non-cardiac deaths (urosepsis, pulmonary embolism, and microcellular pulmonary cancer).
DUPLEX ULTRASOUND
Preprocedural peak systolic velocity (PSV) was 3.68 (2.66, 4.50) m/s and end-diastolic velocity (EDV) was 1.10 (0.80, 1.60) m/s. At 30 days, PSV and EDV were normal12 at 0.60 (0.47, 0.80) m/s and 0.15 (0.12, 0.22) m/s, and remained overall normal at 12 months – PSV 0.74 (0.53, 0.98) m/s and EDV 0.20 (0.14, 0.27) m/s (p=0.14). PSV data are shown in Figure 1, inclusive of the single in-stent restenosis. The isolated restenosis (PSV of 3.9 m/s) was asymptomatic and was treated uneventfully using a drug-eluting balloon. No stent thrombosis occurred throughout 12 months (0%).
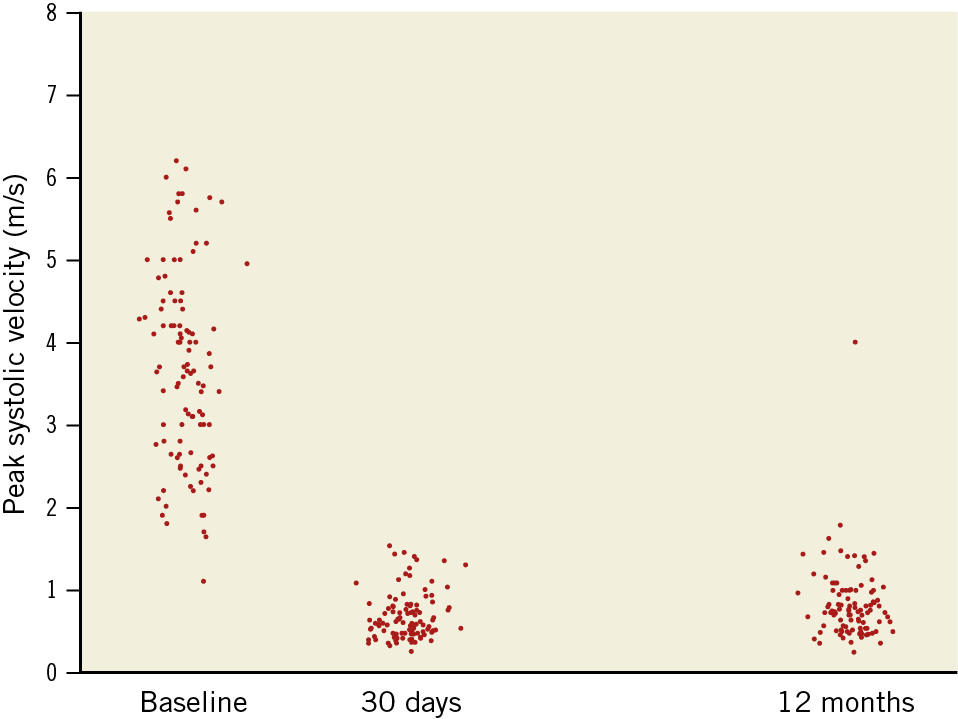
Figure 1. Peak systolic velocity prior to CGuard CAS, and at 30 days and 12 months after the procedure. Individual patient/artery data for all study subjects.
Prior to CAS, 6/106 (5.6%) external carotid arteries (ECAs) were occluded on the target lesion side, whereas 3/100 (3.0%; severe ECA stenosis prior to CAS in all) occluded at CAS. No ECA occlusion occurred between CAS and 30 days and there was no new ECA occlusion at 12 months (post-procedural ECA occlusion rate 0%).
Discussion
Midterm results of our routine use of the novel MicroNET-covered embolic prevention stent (i) show lack of procedure- and/or device-related adverse clinical events between 30 days and 12 months, and (ii) indicate an effective protection against ipsilateral stroke extending post-procedurally.
Normal overall in-stent velocities at 30 days and 12 months (Figure 1), and normal ECA patency are consistent with normal healing12 of this particular dual-layer device in the absence of any device thrombosis or any in-stent restenosis excess12,13,14.
Further discussion is provided in Supplementary Appendix 2.
Limitations
PARADIGM is a single-centre study indicating that larger patient series and extended follow-up are required. The multicentre, multi-specialty PARADIGM study extension (PARADIGM-Extend, ClinicalTrials.gov Identifier: NCT04271033) is continuing enrolment up to its (close) target of 555 consecutive patients.
With a protocol-mandated bias towards the endovascular route using (and challenging) the study device15 in symptomatic and increased-stroke-risk patients in the absence of the “entry” bias of randomised studies15, this investigation differs fundamentally from typical series with symptomatic and/or higher-risk lesions being treated preferentially using CEA16,17.
Further limitations are discussed in Supplementary Appendix 3.
Conclusion
Clinical and DUS data from this symptomatic and increased-stroke-risk consecutive patient series are consistent with the MicroNET-covered carotid stent providing effective protection against cerebral events which extends post-procedurally and with the normal healing profile of the device.
|
Impact on daily practice A low (<1%)5,7,10 periprocedural complication rate with routine use of the MicroNET-covered carotid stent in cohorts including largely symptomatic or increased-stroke-risk clinically asymptomatic carotid stenosis patients is critically important, shifting the carotid revascularisation paradigm13,14. One fundamental message from the present PARADIGM study update is the durability of clinical results for at least one year without any significant restenosis and without any late thrombosis. |
Funding
Jagiellonian University Medical College (ZDS/007819).
Conflict of interest statement
P. Musialek reports being on the speaker bureau, proctoring, consulting, research support, and being on the advisory board of Abbott, InspireMD and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

