
Abstract
Aims: The aim of this study was to evaluate the 30-day clinical outcome of treatment using the Roadsaver carotid stent in non-consecutive subjects at high risk for carotid endarterectomy requiring revascularisation.Methods and results: The CLEAR-ROAD study is a prospective, multinational, single-arm, physician-initiated study planned to include 100 patients in nine centres in Belgium, Italy and Germany. The primary endpoint was the 30-day rate of major adverse events (MAE), defined as the cumulative incidence of any death, stroke or myocardial infarction (MI). The use of embolic protection devices (EPDs) was not mandatory; 31.0% of the patients were symptomatic and in 58.0% of the patients EPDs were used. Technical success was achieved in all cases. The 30-day MAE rate was 2.1% (one patient experienced MI followed by death; another patient experienced a stroke within the first 30 days after procedure). While no statistical analysis could be performed, subgroup data suggested that there were no notable differences in the 30-day MAE rate between symptomatic and asymptomatic patients, or between EPD use.
Conclusions: The 30-day clinical outcome of 100 patients treated with a dual layer micromesh carotid stent (Roadsaver) shows promising results. The Roadsaver stent is a safe and effective device for endovascular treatment of subjects at high risk for carotid endarterectomy.
Abbreviations
CAS: carotid artery stenting
DUS: duplex ultrasound
EPD: embolic protection device
ISR: in-stent restenosis
MAE: major adverse events
MI: myocardial infarction
SAE: serious adverse events
SD: standard deviation
TLR: target lesion revascularisation
Introduction
Carotid artery stenting (CAS) is being widely performed in patients with symptomatic and asymptomatic carotid stenosis at high risk for carotid endarterectomy. A study with 1,453 patients showed that stenting was non-inferior to endarterectomy with regard to the primary composite endpoints (death, stroke or myocardial infarction [MI]). Within the first 30 days after the procedure, the event rate was 3.8% and 3.4% for the stenting and endarterectomy groups, respectively. From 30 days to 5 years after the procedure, there was no difference in the rate of freedom from ipsilateral stroke between the stenting and endarterectomy groups1. In another study with 2,502 patients, the risk of the composite primary outcome of stroke, MI or death did not differ significantly between the group undergoing CAS and the group undergoing carotid endarterectomy. During the periprocedural period, there was a higher risk of stroke with stenting and a higher risk of MI with endarterectomy2. The safety and efficacy of CAS has been well demonstrated in multiple trials3-5 and accepted as an alternative therapy to the surgical approach in multidisciplinary guidelines2,6. With the advances in CAS device technologies, a variety of products with different properties is available to contemporary CAS operators. The stent’s scaffolding capacity plays a major role in preventing procedural events7. To date, the ideal stent design has been discussed, yet remains unclear. In a study with 30 patients, the feasibility of the novel MicroNet™-covered nitinol carotid embolic protective stent system CGuard™ (InspireMD, Boston, MA, USA) was evaluated. Embolic protection devices were used in all procedures. The 30-day major adverse cardiac or cerebrovascular events rate was 0%. New ipsilateral ischaemic lesions at 48 hrs occurred in 37.0% of the patients and the average lesion volume was 0.039±0.08 cm³. The 30-day diffusion-weighted magnetic resonance imaging showed complete resolution of all but one periprocedural lesion and only one new minor lesion in relation to the 48-hr scan8.
The Roadsaver™ carotid stent (Terumo Corp, Tokyo, Japan) presently under investigation is a novel self-expanding stent with a dual layer tubular nitinol mesh, designed to provide sustained embolic protection by extensive plaque coverage and prevention of plaque prolapse. This dual layer stent has a 450 µ lattice that makes it unique in comparison to the other commercially approved carotid stent devices. Hopf-Jensen et al (2015) investigated the Roadsaver stent in a small study with seven patients. They concluded that the Roadsaver stent seems to be safe and effective in the treatment of extracranial internal carotid artery (ICA) stenosis and in the context of tandem lesions in ischaemic stroke9.
Another study investigated the mechanical and implant behaviour of the CASPER-RX stent (MicroVention, Tustin, CA, USA) in 12 patients. They concluded that this stent showed a safe implantation behaviour without the occurrence of any ischaemia. The structure of the new CASPER-RX stent creates an acceptable flexibility, low radial force and high collapse pressure10. CASPER is another brand name for the dual layer micromesh carotid stent (identical to Roadsaver), used for commercialisation within the neuroradiology discipline.
The CLEAR-ROAD (Physician-initiated Carotid Trial Investigating the Efficacy of Endovascular Treatment of Carotid Arterial Disease With the Multi-layer RoadSaver Stent) trial aimed to evaluate the clinical outcomes of treatment by means of stenting with the Roadsaver carotid stent in subjects at high risk for carotid endarterectomy requiring carotid revascularisation due to significant extracranial carotid artery stenosis. This report presents the 30-day outcomes of all 100 included patients.
Methods
STUDY AND DESIGN
The CLEAR-ROAD study is a prospective, multinational, single-arm study in high surgical risk patients with severe carotid stenosis. A total of 100 non-consecutive patients were enrolled in nine clinical centres in Belgium, Germany and Italy between July 2015 and February 2016. An overview with enrolments per centre is shown in Figure 1. The study protocol was approved by the institutional review boards at each participating centre and complies with the Declaration of Helsinki. Written informed consent was obtained from all patients before participation. The inclusion/exclusion criteria are described in Table 1 and Table 2. Patients were selected based on the investigator’s assessment and evaluation as to whether they were at high risk for surgery. The criteria for high surgical risk are listed in Figure 2; however, descriptions of these criteria are not available in this manuscript. A patient was considered to be enrolled after fulfilment of all inclusion/exclusion criteria and successful guidewire passage through the study target lesion. Neurological evaluation was conducted by an independent individual certified to perform NIH Stroke Scale assessment before procedure, at discharge, and at 30-day, 6-month, and 12-month follow-up visits.
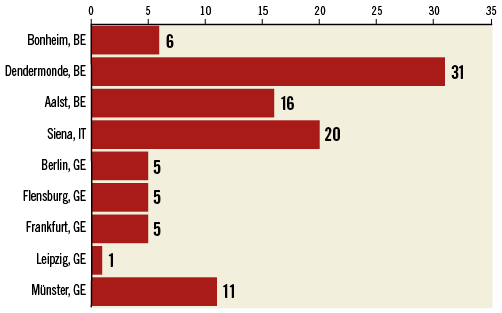
Figure 1. Enrolments per site.
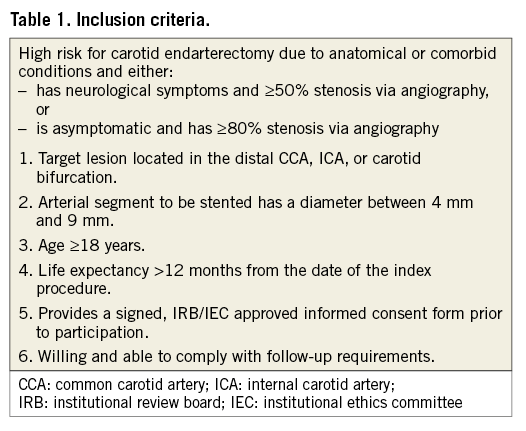
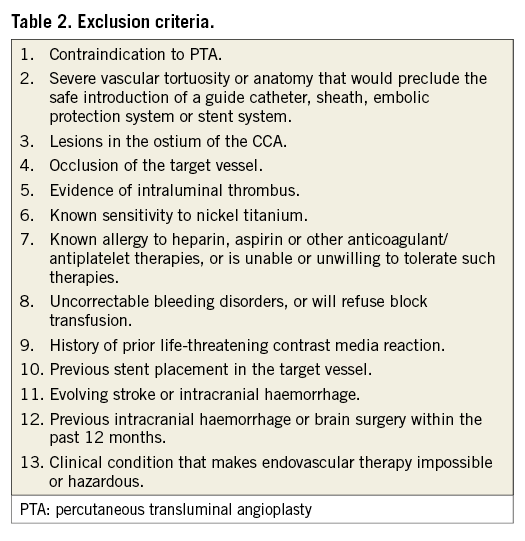
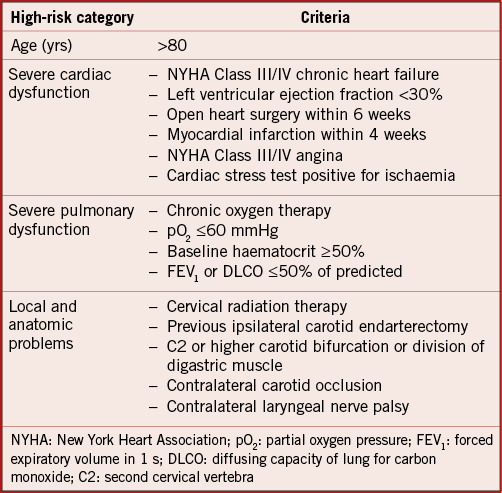
Figure 2. High surgery risk criteria.
THE ROADSAVER CAROTID STENT SYSTEM
The Roadsaver system consists of a self-expanding nickel titanium (nitinol) stent implant and a rapid exchange delivery catheter with 5.0 Fr system compatibility. The implant is produced with various outer diameters ranging from 5-10 mm and with lengths of 20-40 mm. The nitinol stent is constructed from two layers of tubular nitinol mesh. The outer layer consists of a woven closed-cell structure with flared ends. The inner layer consists of a braided closed-cell structure with micro-sized pores (0.381 mm2).
PROCEDURE
The CAS procedure was performed according to the physician’s standard of care. Standard procedures were followed based on the instructions for use of the Roadsaver carotid stent system. Although it was the routine of the services to use EPD in all carotid cases, some investigators felt more confident not using EPD with this type of stent. The vascular access, the use and the type of embolic protection device during the procedure were left to the discretion of the operator. Use of an embolic protection device was not mandatory. Concomitant medication during hospital stay and follow-ups was recommended to be clopidogrel 75 mg daily for one month and aspirin 75-300 mg daily lifelong.
FOLLOW-UP
All participants were asked to visit centres at 30 days after the index procedure; six and 12-month follow-up visits were scheduled. At each visit, carotid duplex ultrasound (DUS), neurological assessment, medication registration, physical examination and adverse event recording were routinely conducted. No specialised core lab analysis was performed in this study.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint was the 30-day rate of major adverse events (MAE), defined as the cumulative incidence of any death, stroke or MI within 30 days post procedure. The secondary endpoints were system technical success, device malfunctions, serious device-related and procedure-related adverse events (SAE), target lesion revascularisation (TLR), in-stent restenosis (ISR), late ipsilateral stroke from 31 days to 1 year, MAE by subgroups (symptomatic status and the use of embolic protection). System technical success was defined as successful deployment of a Roadsaver carotid stent in the target lesion and the successful removal of the delivery system.
STATISTICAL ANALYSIS
Descriptive data were reported as mean ± standard deviation (SD) for continuous variables, or as numbers and percentages for categorical variables. For all time-dependent events, life tables were calculated using Kaplan-Meier estimates. Statistical significance was defined as p-values <0.05. All statistical analyses were performed using SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
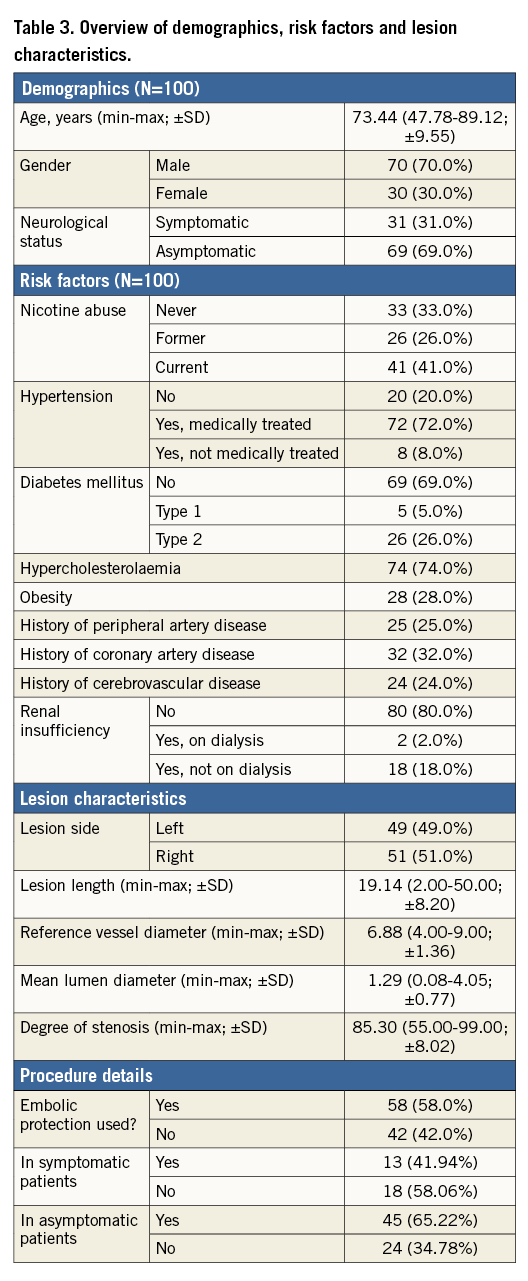
The descriptive data of the 100 patients enrolled indicate a mean age of 73.44 years (range 47.78-89.12; SD ±9.55); 70.0% were male and 31.0% were symptomatic. Symptomatic was defined as patients who had a transient ischaemic attack (TIA) and/or (minor) stroke within 30 days before enrolment. The most prominent risk factor was hypertension (80.0%), followed by hypercholesterolaemia (74.0%), former or current nicotine abuse (67.0%) and history of coronary disease (32.0%). Mean fluoroscopy time was 9.25 minutes (range 0.28-38; SD ±4.97), mean procedural time was 38.73 minutes (range 18-72; SD ±12.25) and post-procedural residual stenosis was 5.21% (range 0-40; SD ±7.40). Mean lesion length was 19.14 mm (range 2-50 mm; SD ±8.20). The reference vessel diameter was defined as the lumen distal diameter + lumen proximal diameter, divided by 2. Mean reference vessel diameter was 6.88 mm (range 4-9 mm; SD ±1.36), mean lumen diameter was 1.29 mm (range 0-4 mm; SD ±0.77) and mean preoperative degree of stenosis was 85.30% (range 55-99%; SD ±8.02). The decision to use an embolic protection device (EPD) was left to the discretion of the operator. There were no guidelines in the protocol as to which type of lesion should be treated with or without the use of an EPD. An EPD was used in 58% of the cases. Of the nine participating centres in this study, four centres decided not to use EPD in some or all of their cases. Of the 58 EPDs used, 48 were distal filters and 10 were proximal occlusion EPDs. For the symptomatic group, 58.06% were treated without the use of an EPD. For the asymptomatic group, 34.78% were treated without the use of an EPD. A more detailed overview of demographics, risk factors, lesion characteristics and procedure details can be found in Table 3. In 21% of the procedures predilatation was performed. Successful stent placement was achieved in all cases (100%). Post-dilatation was performed in 94.0% of the cases. Mean post-dilatation balloon diameter was 5.08 mm (range 3-8; SD ±0.91). The mean maximum pressure was not captured. Within the first 30 days of follow-up, one patient suffered a myocardial infarction (MI) which led to death. One patient suffered a minor ipsilateral stroke (14 days post procedure), due to an atrial fibrillation which was not adequately treated with warfarin medication. Therefore, the freedom from MAE was 97.9%. This resulted in an MAE rate of 2.1%. While no statistical analysis could be performed, subgroup data suggested that there were no notable differences in the 30-day MAE rate between symptomatic and asymptomatic patients, or between EPD use. In symptomatic patients who were treated without an EPD, the freedom from MAE rate at 30 days was 94.4%. In asymptomatic patients who were treated without an EPD, the freedom from MAE rate at 30 days was 100.0%. Standard DUS was performed by the investigators. Although no core lab analysis was performed, the investigators stated in the study source that they did not see any >50% restenosis rate within the implanted Roadsaver stent at the 30-day duplex images. No target lesion revascularisation or loss of primary patency was reported within the first 30 days after the procedure.
Discussion
The 30-day CLEAR-ROAD study results indicate that CAS by means of a dual layer micromesh carotid stent (Roadsaver) is a safe and effective alternative treatment for patients considered at high risk for carotid endarterectomy. One of the risks associated with CAS is the periprocedural stroke resulting from embolisation of debris released during or after the treatment of the carotid stenosis.
To decrease the risk of procedural stroke, several distal protection devices have been introduced. These devices allow the capture and retrieval of friable, lipid-rich plaque. Advantages and disadvantages of several EPDs (balloon occlusion devices, filter devices and catheter occlusion devices) have been described11. In this study a slightly higher number of patients were treated with a protection device. In 42% of the cases, no protection device was used. The fact that in our study the MAE rate between patients treated with and without an EPD had the same trend might raise again the discussion on the necessity to use EPDs. Of course, due to the limited number of patients and lack of randomisation, this study report lacks power and should rather be considered hypothesis-generating, encouraging a further study. The importance of the use of EPDs in combination with this new generation of carotid stent can be discussed, as several reports have pointed out that EPDs do not completely eliminate the risk of cerebral embolisation12,13. Recent studies have postulated that the stent design appears to have a more important impact on the resulting complications in CAS than in EPDs14,15. The majority of the MAE (57.7% in the Capture Registry)16 occur after the procedure (24 hrs) when the filter is removed and the implanted stent is your only “protection device”. The design and the material of carotid stents take on considerable importance in achieving a good long-term clinical outcome. Requirements of the stent are reliable plaque coverage, accompanied by good wall adjustment through high flexibility and radial force17,18. Several experimental and clinical studies have demonstrated the advantage and superiority of the closed-cell design regarding the complication and outcome results due to CAS19.
Improvements in stent design and technical skills nowadays make CAS, even without the use of an EPD, an effective and safe therapy for symptomatic and asymptomatic stenosis of the carotid artery20.
Limitations
Limitations in this study are the small number of patients, not including diffusion-weighted imaging (DWI) acquisition and the fact that there was no randomisation between a group treated with the use of an EPD and a group treated without the use of an EPD. Also, external carotid artery (ECA) flow/patency was not captured in this study. The total number of CAS procedures performed in the participating centres during the duration of this study was not captured. It would be interesting to compare the older-generation carotid stents with the newer-generation carotid stents (with enhanced scaffold capacities) in a randomised controlled trial.
Conclusions
The 30-day clinical outcome of 100 patients treated with a dual layer micromesh carotid stent (Roadsaver) shows promising results. The Roadsaver stent is a safe and effective device for endovascular treatment of subjects at high risk for carotid endarterectomy.
| Impact on daily practice The use of the Roadsaver stent can be a valid alternative for the treatment of carotid artery lesions in symptomatic and asymptomatic patients with a high risk for surgery, and could be incorporated into daily practice. |
Funding
This study is an investigator-initiated trial, funded by Terumo.
Conflict of interest statement
S. Müller-Hulsbeck has received speaker’s fees from Terumo Europe, and grants for Terumo carotid workshops. D. Scheinert is on the advisory board of and/or is a consultant for Abbott, Biotronik, Boston Scientific, Cook Medical, Cordis, CR Bard, Gardia Medical, Medtronic/Covidien, TriReme Medical, Trivascular, Upstream Peripheral Technologies. H. Sievert has received study honoraria, travel expenses, consulting fees from Abbott, Acoredis, Atrium, Biosense Webster, BioVentrix, Boston Scientific, Carag, Cardiac Dimensions, CardioKinetix, Celonova, CGuard, Coherex, Comed B.V., Contego, CSI, CVRx, ev3, FlowCardia, Gardia, Gore, GTIMD Medical, Guided Delivery Systems, Hemoteq, InspireMD, Kona Medical, Lumen Biomedical, Lifetech, Medtronic, Occlutech, pfm medical, Recor, SentreHeart, Svelte Medical Systems, Terumo, Trivascular, Valtech, Vascular Dynamics, Venus Medical, and Veryan, and has stock options for CardioKinetix, AccessClosure, Coherex, and SMT. The other authors have no conflicts of interest to declare.

