
With the long-term equipoise of carotid artery stenting (CAS) and carotid endarterectomy (CEA) demonstrated unequivocally in CREST and other large studies, the debate between CAS and CEA today is about the (mostly minor) strokes within the first 30 days after the procedure. With conventional (single-layer) carotid stents, the risk for cerebral embolisation (unlike in CEA) continues post-procedurally1, i.e., when the embolic protection device is no longer able to capture the debris. As 40-80% of strokes associated with conventional-stent CAS are post-procedural, minimisation of periprocedural embolism and elimination of post-procedural embolism are critical for the future of CAS2.
With conventional carotid stenting, plaque prolapse3-5 – a major mechanism of post-procedural stroke5 – occurs via the “cheese-grater” effect, in a phenomenon similar to that observed with single-layer coronary stents6. To address the problem of plaque prolapse in CAS, mesh-covered carotid stents have been developed and introduced into routine clinical practice2,7. The “ultra-closed cell” carotid stent design is produced by covering the nitinol frame with a mesh that can be made of different materials7-9. At present, the two mesh-covered stents in clinical use are RoadSaver® (aka Casper; MicroVention/Terumo [Tokyo, Japan])8 and CGuard™ EPS (InspireMD, Boston, MA, USA)9,10. Apart from the differences in the nitinol frame (braided closed cell in RoadSaver/Casper7,8, laser-cut open cell in CGuard7,9), the devices have three important differences in relation to the mesh coverage: (i) the mesh material is different (braided nitinol in RoadSaver/Casper, PET Micronet in CGuard EPS); (ii) the mesh pore aperture size is different (375-500 µm in RoadSaver/Casper vs. 150-180 µm in CGuard EPS); and (iii) the position of the mesh in relation to the nitinol frame is different (outside the frame for the CGuard EPS, and inside in the case of the RoadSaver/Casper)7-9. Indeed, with the MicroNet covered carotid stent systematic per protocol DW-MRI evaluation demonstrated minimisation of intraprocedural embolisation and elimination of post-procedural cerebral embolisation10. This strategy has been termed intraprocedural and post-procedural (sustained) “embolic prevention” in CAS10,11.
In this issue of EuroIntervention, Umemoto and colleagues12 report outcomes of CAS patients treated with two different mesh-covered stents.
Endpoints of interest included the occurrence of stent malapposition (defined as at least five malapposed struts in a single OCT slice and with the strut defined as “malapposed” when the distance between vessel wall and the strut surface exceeded 200 µm) and plaque prolapse (defined as tissue protrusion more than 300 µm from the stent strut level). On slice-based analysis, stent malapposition occurred in 26.8% with RoadSaver vs. 20.5% with CGuard EPS (p=0.26) and plaque prolapse in 20.7% and 10.8%, respectively (p=0.05).
The messages from the work by Umemoto and colleagues12, indicating a similar rate of stent malapposition with the two designs and an approximately twofold greater risk of residual plaque prolapse with the dual metallic layer device, deserve further investigation. This is not only because, ideally, one would want to see a larger study in patients with well-defined baseline plaque characteristics6, but also because this work illustrates some fundamental limitations of the current definitions and techniques when used to evaluate the novel devices. For stent malapposition, in an appropriately sized open-cell stent9,11, malapposition may be minimised by optimising post-dilatation. This, however, is more difficult to achieve with the dual-layer braided closed-cell design of the nitinol stent frame8. Stent malapposition will depend on stent expansion optimisation in the context of stent design (open vs. closed-cell frame) and the stent nominal diameter in relation to the vessel diameter. Initial data indicate that open-cell mesh-covered stents may allow more robust optimisation11.
It is important to realise that the differences in design of the two devices8,9, including the mesh material and mesh position in relation to the stent frame, translate into completely different types of plaque prolapse. In RoadSaver/Casper, the braided metallic mesh of 45 µm-diameter of nitinol filament is fixed (at many fixation points8) inside the braided stent frame and, because its diameter significantly exceeds the axial resolution of optical coherence tomography (OCT), the mesh is routinely visualised on OCT (see Figure 6A in ref. 12 and the schematic presentation in Figure 1 below), and the OCT-detected plaque prolapse12 represents atherosclerotic plaque that is not mesh-covered (schematic presentation in Figure 1A below, for raw OCT images see Figure 6B in ref. 12). In contrast, in the CGuard EPS, the mesh, that is fixed to the stent frame only at its edges, can adapt flexibly to the plaque prolapse through the highly open cells of the CGuard EPS stent frame, with mesh coverage of the plaque protruding into the vessel lumen (see Figure 1A below, compare with a raw OCT image in Figure 7 in ref. 12). As found by Umemoto and colleagues12, conventional OCT may, in some slices, indicate mesh coverage of the prolapsing atherosclerotic plaque in CGuard EPS (cf. Figure7 in ref. 12). Indeed, mesh coverage of protruding plaque prevents embolism by “trapping” the plaque in situ. However, we cannot be absolutely sure that prolapsing plaque is mesh covered – the CGuard EPS mesh is made of PET fibre that is only 20 µm in diameter9, which is too small to permit systematic visualisation on conventional OCT (Figure 1C, Figure 1D).
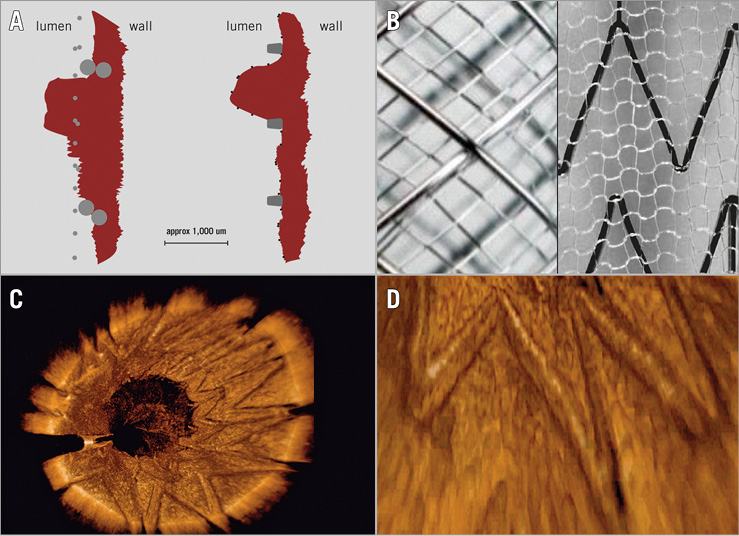
Figure 1. Residual plaque prolapse through mesh-covered carotid stent types in the context of fundamental stent design differences. A) Schematic presentation of plaque prolapse (PP). Left: schematic representation of a carotid stent with metallic mesh made of braided nitinol filaments 47 µm in diameter (filament-to-filament distance of ca. 375 µm) fixed (with fixation points along the stent length)8 inside the stent frame that is made of braided nitinol wires of 180 µm. Right: schematic representation of a carotid stent with PET-fibre MicroNet, positioned outside the stent frame and fixed to the frame only at the proximal and distal stent edge9 that has the ability to adapt to the plaque, covering any protrusion (cf. Figure 7 in ref. 12) that may occur between the widely open laser-cut nitinol struts of 92×125 μm. The PET-fibre diameter is only 20 µm – beyond the effective resolution of conventional OCT. Nitinol is shown in grey, PET in black, and the plaque in dark red. B) Photographs (abluminal view) of the two dual-layer carotid stent designs available for commercial use today. Left: RoadSaver/Casper (braided nitinol closed-cell frame and braided nitinol closed-cell mesh that is fixed inside the stent frame). Right: CGuard EPS (laser-cut open-cell nitinol frame with knitted PET MicroNet outside the stent frame). According to refs 7 and 9, modified. C) 3D OCT reconstruction of the CGuard EPS implanted into a symptomatic human carotid artery. Note a clear lumen definition in the absence of PP (minor opacifications in the lumen are artefacts from residual contrast agent). According to ref. 7, modified. D) Detailed section from panel C. Note that the MicroNet PET fibres (20 µm in diameter) are beyond the effective resolution of conventional OCT; thus, the mesh is only barely indicated behind the stent frame (i.e., between the stent frame and the vessel wall). According to ref. 7, modified.
Unfortunately, the findings of Umemoto and colleagues12 cannot be directly compared with the recent paper of Nerla et al13, because of the per-slice analysis in one study and the per-patient analysis in the other. Ideally, both per-patient and per-slice data should be provided because each conveys different information, and for future studies it would be valuable if this approach could be standardised. Also, the extent of the plaque prolapse (in terms of its area and “depth”) may confer different risks of cerebral embolism. Further investigation of this issue is a matter of considerable interest.
Future efforts will need not only to evaluate plaque prolapse predictors6 in the novel mesh-covered carotid stents but also to determine whether there is any definable unprotected (i.e., not covered/excluded by micromesh) plaque prolapse threshold for subclinical vs. clinical cerebral embolism. Moreover, rapidly evolving innovations will improve intravascular imaging resolution, enabling routine MicroNet filament visualisation. The research cycle of mesh-covered carotid stents (that now expands to large-scale clinical studies with a long-term follow-up2) may need re-evaluation in animal models, with progress in imaging technologies14,15 in a position to demonstrate unequivocally that the residual plaque prolapse seen with CGuard EPS is indeed mesh covered.
Conflict of interest statement
P. Musialek was co-Principal Investigator in the InspireMD CARENET Trial (Carotid Embolic Protection Using MicroNet) and participated in the CGuard Advisory Board. Currently he is a Principal Investigator in the industry-independent PARADIGM/PARADIGM–Extend Academic Study. E. Stabile has no conflicts of interest to declare.

