
Carotid artery stenting (CAS) is undergoing a new “Renaissance”. Increased operator experience together with advances in technology have provided enough answers to the known controversies traditionally associated with CAS, with a definite improvement in clinical outcomes observed in the latest trials1. The reasons for this deserve an adequate critical appraisal.
It is a fact that carotid angioplasties are emboli-generating procedures. Cerebral embolisation is the main explanation for the post-procedural cerebral events observed after CAS2, with large-burden, friable, thrombotic and ulcerated plaques being associated with a higher risk of embolisation3,4. The use of neuroprotection devices has significantly reduced the risk of embolisation during CAS5. However, the choice of stent is crucial when considering the risk of plaque prolapse and distal embolisation until re-endothelialisation is complete. Treating carotid plaques at high risk of embolisation requires a stent with reliable plaque coverage and long-acting plaque prolapse prevention. Consequently, the decision as to the right stent in the right patient always needs to balance the need for plaque scaffolding and the presence of an adequate conformability to each different anatomical configuration (Figure 1).
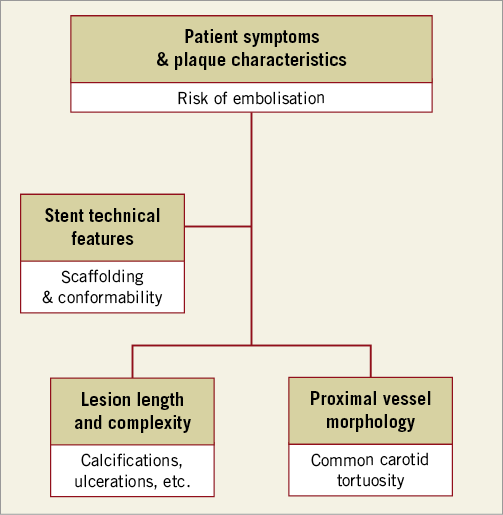
Figure 1. A proposed algorithm showing the ideal characteristics of the stents to be deployed during CAS according to the individual patient’s vascular anatomy and lesion morphology.
Dealing with high-risk plaques, a clear benefit in favour of stent scaffolding in order to reduce post-procedural neurological complications was first demonstrated by Bosiers et al6. Furthermore, in recent years the use of double-mesh technology stents has been able to abolish the risk of periprocedural clinical events completely in patients undergoing CAS, both in clinical trials and in real-world registries7-9.
In the current issue of EuroIntervention, Speziale et al10 report their experience in a real-world registry of patients undergoing protected CAS with the CGuard™ Embolic Prevention System (InspireMD, Boston, MA, USA).
They showed only minor neurological complications in the periprocedural phase and no neurological events at all at 30-day follow-up. In addition, diffusion-weighted magnetic resonance imaging (DW MRI) showed only a minor number of lesions with no clinical relevance. Although obtained in a relatively low-risk population of asymptomatic patients with all types of lesion, the study offers the umpteenth confirmation that cerebral protection obtained by integrating an embolic protection device and a stent with excellent plaque coverage is associated with no neurological events in the conventional 30-day follow-up after CAS. Of note, micro-mesh (MicroNet™) CGuard technology was used as a workhorse tool in routine CAS practice, with only one patient being excluded from the registry due to an extremely tortuous vascular anatomy not considered suitable for the stent.
The present report, together with the growing literature about double-mesh stents, perfectly illustrates how the scenario of CAS has actually changed. With the procedural and 30-day risk further confirmed to be <1%, significantly lower than the five-year risk associated with the natural history of the disease, a decision on whether to offer intervention on top of optimal medical therapy should be considered in the earlier phases, when the natural history of the disease (i.e., asymptomatic status) has not yet irreversibly compromised the patient’s quality of life.
Apart from raising interest in CAS, this advance should also highlight the need for a quality control of the procedures. Technological advances, including mesh-covered carotid stents for sustained embolic prevention and temporary protection devices, should always be evaluated in the context of a standardised and tailored approach for each different patient and each different lesion. With this aim in mind, operators’ experience and training are crucial, both in patient/lesion selection and in appropriate device choice. The unique opportunity of having a completely “protected” procedure, which extends up to 30 days from the stent deployment, is now possible thanks to developments in stent technology and definitely demands that CAS procedures be performed by experienced and trained operators tailoring their strategy to patients’ anatomical and clinical characteristics.
Conflict of interest statement
The authors have no conflicts of interest to declare.

