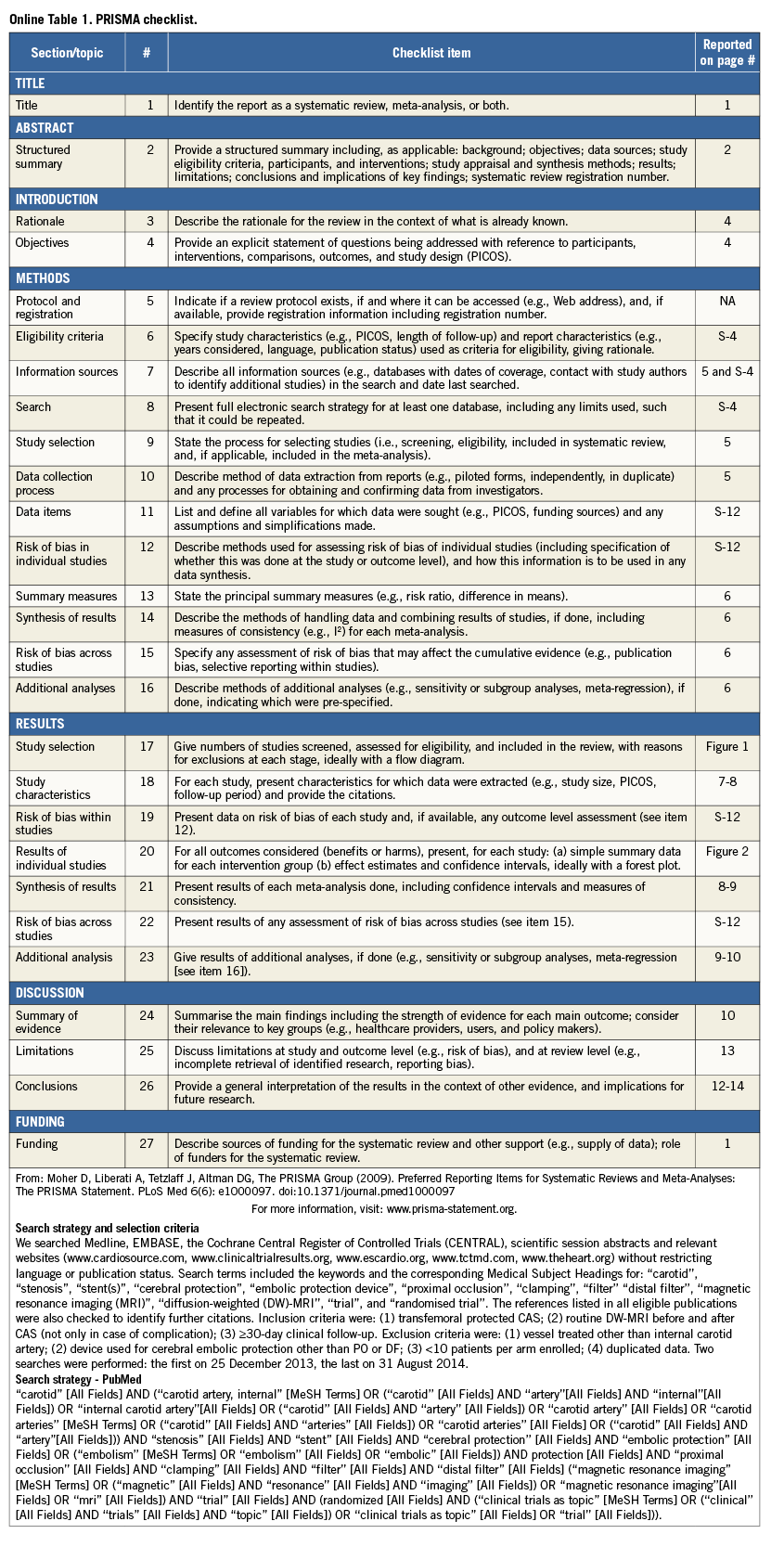
Abstract
Aims: Proximal occlusion (PO) and distal filter (DF) serve for cerebral embolic protection during carotid artery stenting (CAS). New cerebral lesions at diffusion-weighted magnetic resonance imaging (DW-MRI) represent a surrogate endpoint for embolisation, though their clinical impact is controversial. We performed a meta-analysis of randomised and observational DW-MRI studies comparing PO and DF during CAS.
Methods and results: We searched electronic scientific databases. The primary endpoint was the incidence of new cerebral lesions at DW-MRI; secondary endpoints were the incidence of new ipsilateral and new contralateral cerebral lesions at DW-MRI and death/cerebrovascular events (CVE). A total of 392 patients (seven studies) received CAS. At DW-MRI after 48 hours 178 patients (48.3%) presented new cerebral lesions. The use of PO versus DF reduced neither the risk of new cerebral lesions (OR [95% confidence interval] 0.65 [0.28-1.52], p=0.32) nor the risk of death/CVE (0.59 [0.22-1.60], p=0.30). Diabetes, baseline stenosis and symptoms significantly modified the risk estimates for new cerebral lesions.
Conclusions: In this meta-analysis, one half of patients receiving protected CAS developed new embolic cerebral lesions at DW-MRI, although the overwhelming majority were asymptomatic. Cerebral protection with PO versus DF neither reduced cerebral embolisation nor impacted on clinical outcomes.
Introduction
In high-volume centres with documented low complication rates, patients with an indication for carotid revascularisation can be successfully treated with percutaneous carotid artery stenting (CAS) without the need for general anaesthesia or neck dissection as compared with carotid endarterectomy1,2. However, the cerebral migration of debris may occur at any stage during CAS and, although often asymptomatic, represents the major drawback of the procedure3,4. Diffusion-weighted magnetic resonance imaging (DW-MRI) is a valuable tool with high sensitivity to identify signs of new cerebral embolisation following CAS. Notwithstanding this, the clinical impact of these cerebral lesions is still controversial5.
Several devices have been developed to prevent cerebral embolisation during CAS, and current guideline-writing authorities suggest that embolic protection should be considered when the risk of vascular injury is low1,2. Cerebral protection during CAS is most commonly accomplished by using either proximal occlusion (PO) or distal filter (DF)6. In the case of PO (with or without extracorporeal arteriovenous shunting) the blood flow of the target carotid artery and the ipsilateral vascular system is reversed through the inflation of balloons placed in the common and external carotid arteries, without crossing the culprit lesion7. In the case of DF a mesh-based basket is advanced through the culprit lesion in the internal carotid artery and then opened distally to entrap particles generated during catheter-based manipulations.
To date, it remains uncertain whether PO or DF is the more effective in reducing the risk of cerebral embolisation during CAS, as investigations on this topic have given inconsistent results8-11.
This meta-analysis of DW-MRI studies compared PO versus DF for cerebral embolic protection during CAS.
Methods
SEARCH STRATEGY AND SELECTION CRITERIA
All details of search strategy and selection criteria are provided in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist (Online Table 1), and this study was performed in compliance with the PRISMA statement12.
DATA COLLECTION AND ASSESSMENT OF RISK OF BIAS
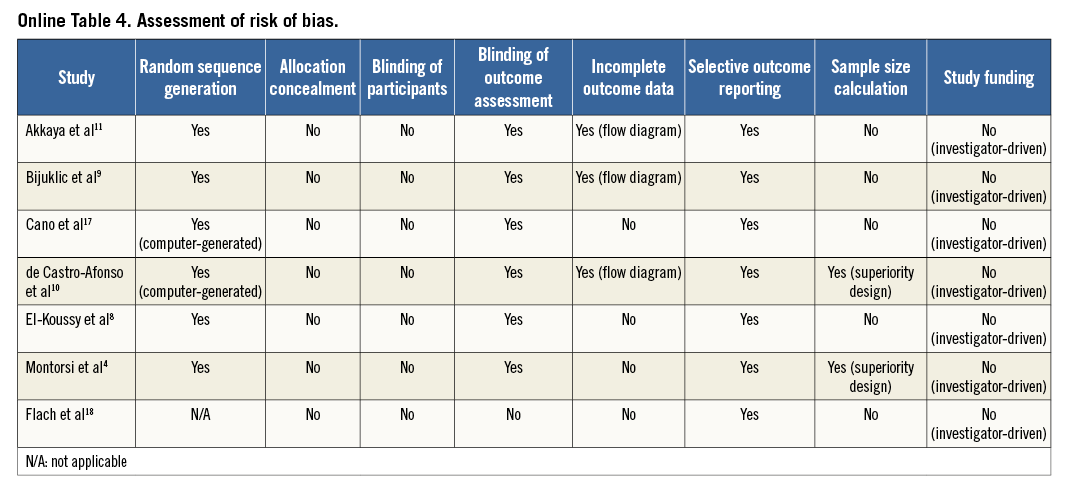
Two investigators (S. Cassese and G. Ndrepepa) independently assessed publications for eligibility at title and/or abstract level, with divergences resolved by a third investigator (M. Fusaro). Studies which met inclusion criteria were selected for further analysis. Freedom from bias was evaluated for each study by the same investigators, in accordance with The Cochrane Collaboration method13. We avoided formal quality score adjudications, which have previously been considered potentially misleading14.
OUTCOME VARIABLES
The primary outcome was the incidence of new cerebral lesions at DW-MRI after CAS. Secondary outcomes were the incidence of new ipsilateral and new contralateral cerebral lesions at DW-MRI after CAS, as well as the incidence of death/cerebrovascular events (CVE). Endpoints were evaluated as per protocol definitions. Where further details were required, we attempted to obtain them from the study investigators directly.
STATISTICAL ANALYSIS
Statistical analysis was performed using the Review Manager Version 5.1 (RevMan; The Cochrane Collaboration, Copenhagen, Denmark), and Stata 11.2 (StataCorp, College Station, TX, USA) software packages.
Distribution of patients and study characteristics were presented as median (interquartile range). Odds ratio (OR) and 95% confidence interval (95% CI) served as summary statistics for comparing PO versus DF devices, and risk estimates were displayed according to study design (randomised/observational). The Mantel-Haenszel random effects model (DerSimonian and Laird) was used to obtain pooled OR. For studies in which only one of the treatment groups had no events of interest, the risk estimates were approximated from 2×2 contingency tables after adding 0.5 to each cell15. The Breslow-Day χ2 test and the I2 statistic were used to test heterogeneity across the studies13, whilst the restricted maximum likelihood method (Tau2) tested between-study heterogeneity. Visual estimation of funnel plot as well as statistical tests assessed possible publication bias for the primary outcome, as previously published16. An influence analysis was performed for the primary outcome. A random effects sensitivity analysis evaluated the extent to which several covariates might have influenced the risk estimates for endpoints showing significant heterogeneity. Covariates included: the size of the study (under/above median number of patients enrolled), the enrolment in a centre experienced in CAS procedures (>50/year)1, the type of PO (with/without arteriovenous shunt), the type of DF (concentric/eccentric), the stent design (closed/open cell), the sensitivity of MRI (1.5- or 3-Tesla MRI scanner), the timing of MRI (≤24 or >24 hours after CAS), the age (under/above median value), the average of males (under/above median value), or diabetics (under/above median value), the grade of baseline stenosis (under/above median value) and the average of symptomatic patients (under/above median value). Finally, a random effects meta-regression analysis assessed the relation between new cerebral lesions (expressed as the proportion of patients with new cerebral lesions at DW-MRI after CAS) and the risk estimates for death/CVE.
Results
ELIGIBLE STUDIES
The process of study selection is summarised in Figure 1. Seven studies –six randomised4,8-11,17, one observational18, all full-length manuscripts– were selected for inclusion in the meta-analysis. A total of 392 patients undergoing protected CAS (193 with PO and 199 with DF) were studied.
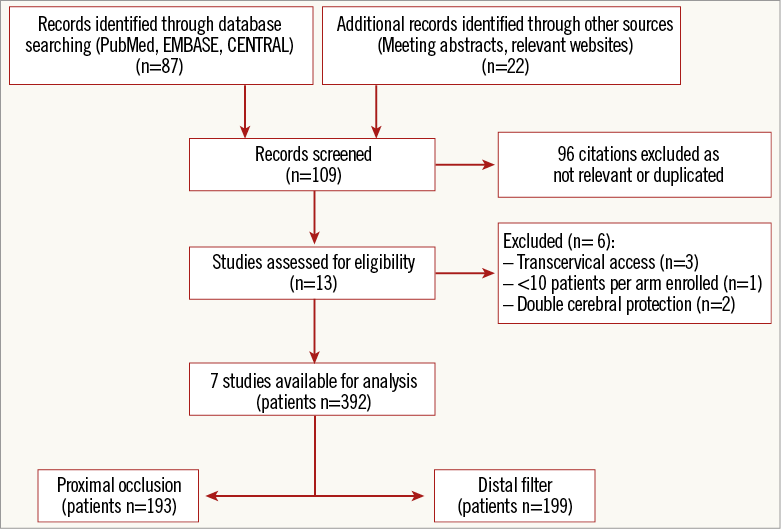
Figure 1. PRISMA flow chart for the study selection process. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses
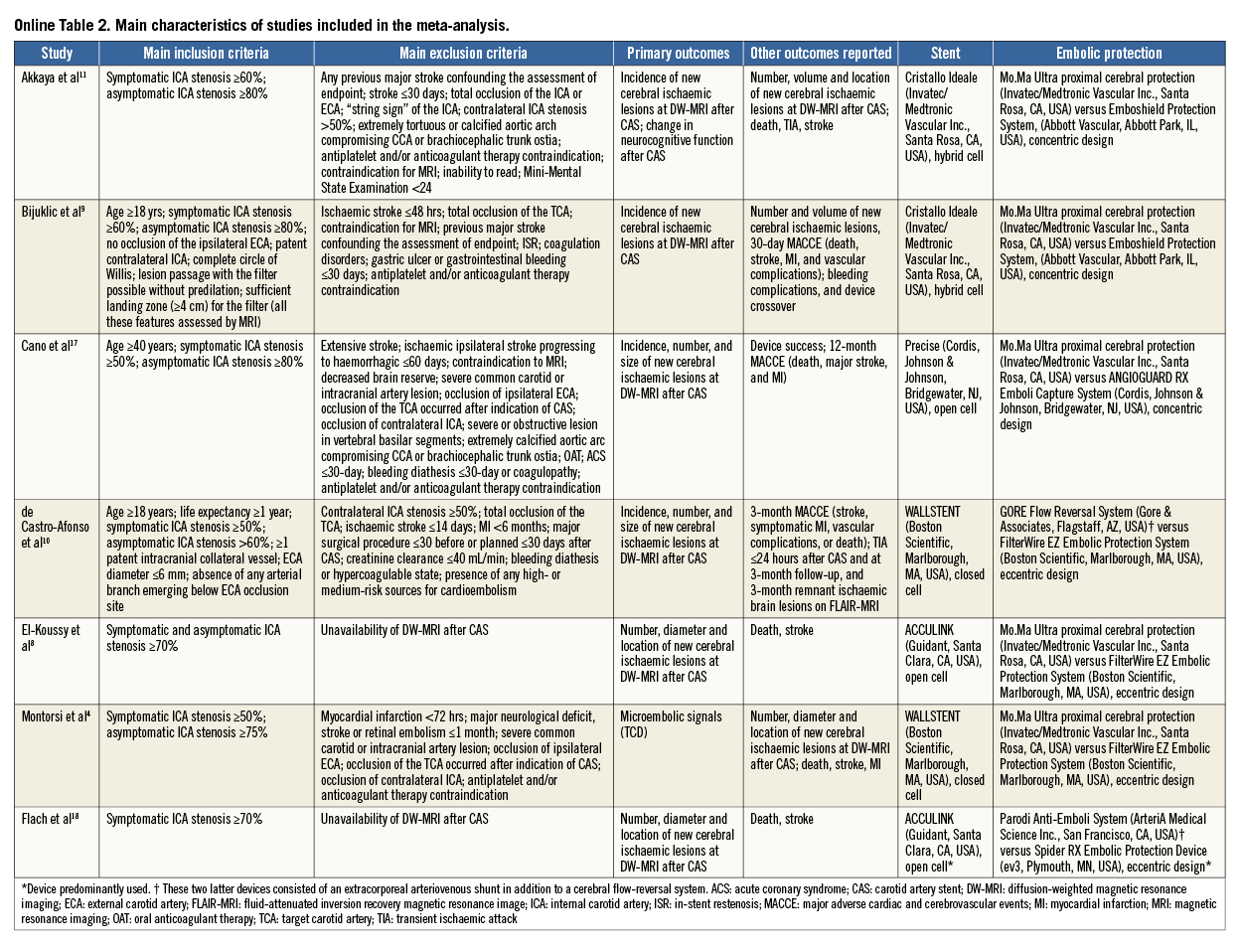
The main characteristics of the included studies, the devices used to accomplish cerebral protection and the types of stent implanted are described in detail (Online Table 2). All studies were single-centre except one11, and all studies reported the institutional level of experience (cases of CAS/year) prior to starting the enrolment. Patients with evidence of ≥50% to ≥70% symptomatic stenosis or evidence of ≥60% to ≥80% asymptomatic stenosis of the internal carotid artery were assigned to transfemoral protected CAS with PO versus DF. Patients with an occluded target carotid artery, recent stroke, contraindication to antiplatelet and/or anticoagulant therapy, common contraindications to MRI (i.e., pacemaker, claustrophobia, etc.) and other possible sources of cerebral embolism were excluded. In one study18, several different distal filters, as well as several different stent types, were used: for the purpose of the present analysis we assumed that the most used were representative of the entire cohort. In one trial, for two patients (0.7% of all patients included in the meta-analysis) carotid intervention consisted of balloon angioplasty only8. Overall, few cases crossed over due to inability to advance the device assigned4.
The median number of patients included in each study was 53 (44-62), and the clinical characteristics matched those of patients suffering from cerebrovascular disease (Table 1). The median age was 68.8 years (67.7-70.1) with a high frequency of males (71% [63-79]), diabetics (29% [25-40]) and high-grade baseline stenosis (85.0% [83.6-86.2]). In detail, patients receiving cerebral protection by means of PO had a baseline stenosis of 86.4% (84.4-88.0), whilst those receiving cerebral protection by means of DF had a baseline stenosis of 82.8% (82.0-86.1).
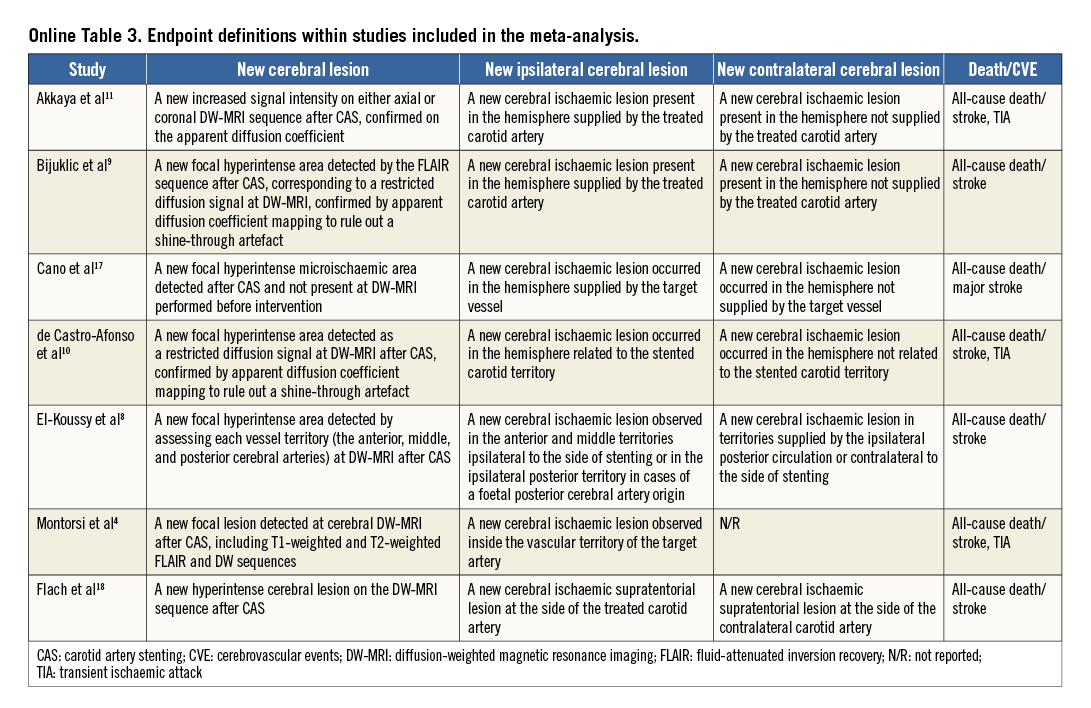
An overview of the definitions of the endpoints in the studies is reported in Online Table 3. In all but one study4, the primary endpoint was the incidence of new cerebral lesions at DW-MRI after CAS. One study4 primarily evaluated the rate of embolic signals at transcranial Doppler echography during protected CAS. Only one study tested the change in the neurocognitive function six months after CAS11. All patients received protected CAS in addition to standard medical therapy. Two studies10,18 administered a 300 mg clopidogrel loading dose before CAS, and dual antiplatelet therapy was prescribed for 30 days after index procedure in all studies but one10. The risk of bias among studies is reported in Online Table 4.
IMAGING AND CLINICAL OUTCOMES
A total of 368 patients (93.8%) received DW-MRI after CAS at 48 hours (24-72). New cerebral lesions at DW-MRI after CAS were observed in 178 patients (48.3%). The use of PO versus DF did not reduce the risk of new cerebral lesions although the heterogeneity was significant (0.65 [0.28-1.52], p=0.32; I2=68%, p for heterogeneity - phet=0.004) (Figure 2A).
New ipsilateral cerebral lesions at DW-MRI after CAS were observed in 161 patients (43.7%). The use of PO versus DF did not reduce the risk of new ipsilateral cerebral lesions although the heterogeneity was significant (0.62 [0.26-1.47], p=0.28; I2=65%, phet=0.009) (Figure 2B).
New contralateral cerebral lesions at DW-MRI after CAS were observed in 44 patients (13.2%; data available for 333 [90.4%] patients, six studies8-11,17,18). The use of PO versus DF did not reduce the risk of new contralateral cerebral lesions without heterogeneity between studies (0.56 [0.28-1.13], p=0.11; I2=0%, phet=0.11) (Figure 2C).
Clinical follow-up was to 135 days (30-360). Death/CVE occurred in 16 patients (4.1%). The use of PO versus DF did not reduce the risk of death/CVE without significant heterogeneity between studies (0.60 [0.22-1.63], p=0.32; I2=0%, phet=0.98) (Figure 2D).
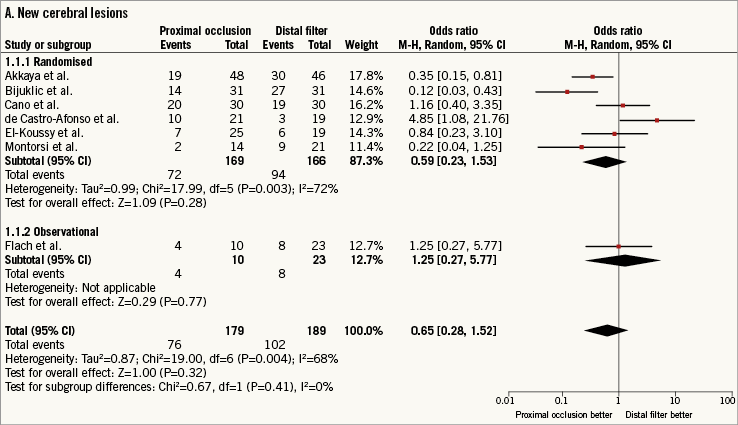
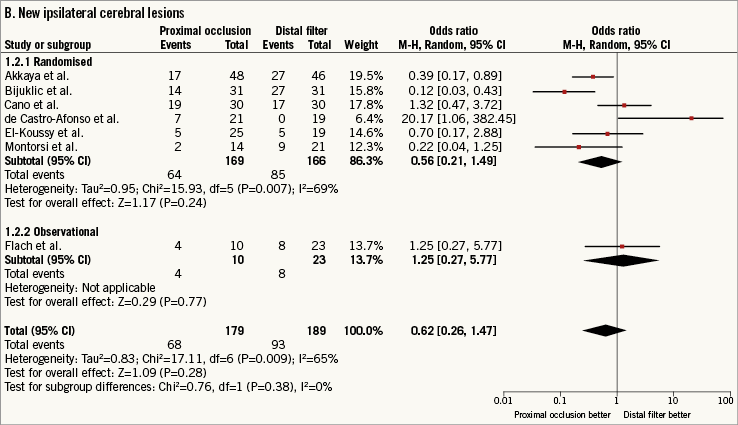
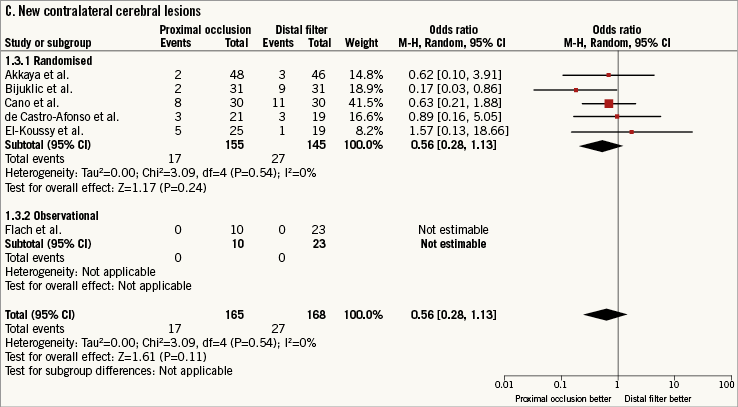
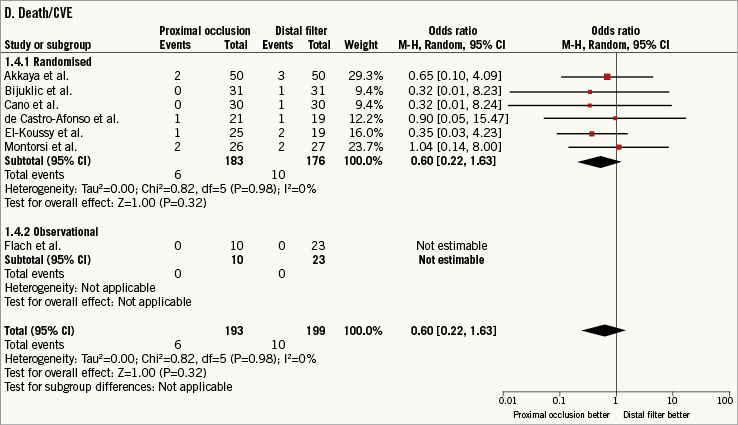
Figure 2. Risk estimates of primary and secondary outcomes for proximal occlusion versus distal filter during carotid artery stenting. Plot of odds ratio for primary (A) and secondary outcomes (B-D) associated with proximal occlusion versus distal filter. The diamond indicates the point estimate and the left and the right ends, the 95% confidence interval (CI). The test for subgroup differences describes the interaction between study design (randomised/observational) and the outcomes (p-value <0.05 indicates significance). CVE: cerebrovascular events; M-H: Mantel-Haenszel
Approximation from 2×2 contingency tables may have a major impact on the results when the outcomes are rare. For this reason, the non-approximated risk of death/CVE was calculated using the exact conditional likelihood method according to Martin and Austin19. The use of PO versus DF did not reduce the non-approximated risk of death/CVE without significant heterogeneity between studies (0.55 [0.20-1.50], p=0.25; I2=0%, phet=0.88).
SMALL STUDY EFFECTS, INFLUENCE AND SENSITIVITY ANALYSES
Funnel plot distribution of primary outcome was derived from the standard error of the logarithm OR plotted against the OR of new cerebral lesions (Online Figure 1). Of note, the absence of bias due to small study effects was confirmed both visually and mathematically. Additionally, the influence analysis demonstrated that no single study significantly altered the summary OR for new cerebral lesions (data not shown).
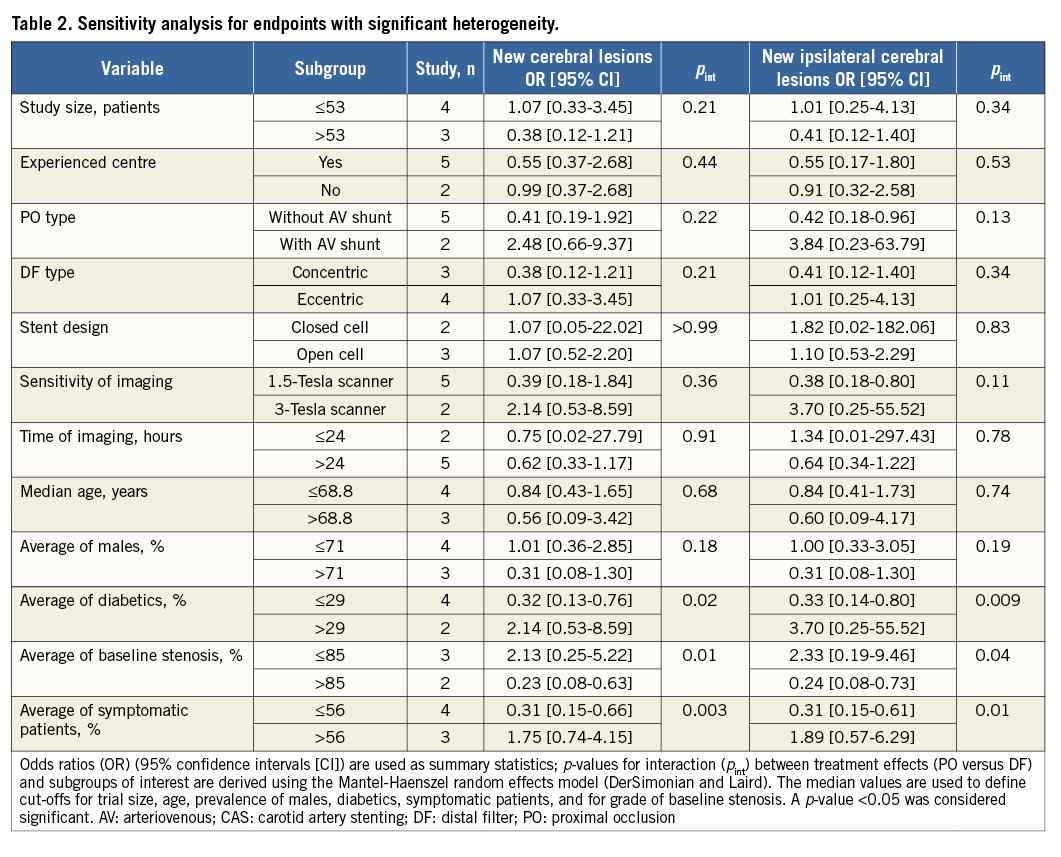
To investigate the sources of the observed heterogeneity, two sensitivity analyses were conducted (Table 2). There was a significant modification of treatment effect for new cerebral lesions by average of diabetics (p for interaction – pint=0.02), high-grade baseline stenosis (pint=0.01) or presence of symptoms (pint=0.003). There was significant modification of treatment effect for new ipsilateral cerebral lesions by average of diabetics (pint=0.009), high-grade baseline stenosis (pint=0.04) or presence of symptoms (pint=0.01). The study size, the centre experience in CAS procedures, the type of PO or DF, the stent design, the sensitivity or timing of DW-MRI after CAS, the age of the patients and the average of males did not modify the risk estimates.
The meta-regression analysis showed no significant relationship between the presence of new cerebral lesions at DW-MRI after CAS and the risk of death/CVE (Online Figure 2).
Discussion
This meta-analysis investigated whether PO versus DF reduces the risk of cerebral embolisation associated with CAS. The main findings can be summarised as follows: (i) at DW-MRI 48 hours after protected CAS one half of patients present new embolic cerebral lesions, though the large majority of events are asymptomatic; (ii) the use of PO versus DF does not influence the risk of new cerebral lesions after CAS with significant modification of treatment effect by diabetes, baseline stenosis and symptoms; (iii) the use of PO versus DF during protected CAS does not impact on the risk of death/CVE.
Patients suffering from atherosclerotic disease of the carotid arteries represent an important challenge due to the high risk of disability and comorbidity20. In recent years, CAS has emerged as a less invasive revascularisation option with similar efficacy compared to surgery in centres performing a large number of procedures1. Although remaining largely asymptomatic, cerebral embolisation associated with CAS represents a matter of concern21. Given the paucity of clinical events after contemporary CAS22, imaging techniques are useful in studies assessing treatment efficacy and safety. In this regard, DW-MRI has shown itself to be a sensitive and reliable tool to identify signs of new cerebral embolisation following CAS23.
Despite the lack of dedicated, large-scale, sufficiently powered, randomised trials favouring protected CAS, guideline-writing authorities suggest that embolic protection should be considered for patients undergoing endovascular carotid revascularisation (Class IIb recommendation)1,2. Several embolic protection devices have been developed to prevent the cerebral migration of debris associated with catheter manipulations during CAS6. Among embolic protection devices, PO and DF are the most commonly used in contemporary practice. Whether the use of PO as compared with DF reduces the risk of cerebral embolisation during CAS is still debated. Indeed, previous small-sized studies found that PO versus DF was associated with a lower4,9,11,17, similar8,18 or higher10 incidence of new cerebral lesions at DW-MRI after CAS.
In the present analysis, we pooled the study-level data of seven studies investigating the efficacy of PO versus DF in reducing the risk of new cerebral lesions associated with CAS. DW-MRI served for the assessment of imaging outcomes. Firstly, this meta-analysis shows that half of patients have new signs of cerebral embolisation at DW-MRI performed within 48 hours after protected CAS. The large majority of these new signs are ipsilateral, do not lead to clinical sequelae and have no significant relationship with the risk of adverse outcomes. These results merit careful discussion.
DW-MRI allows for high sensitivity comparison of embolic protection during CAS. However, the clinical relevance of new asymptomatic cerebral lesions at DW-MRI after CAS represents a matter of ongoing controversy23. The present meta-analysis did not find a relationship between the incidence of new cerebral lesions after protected CAS and the risk of death/CVE. Similarly, a large retrospective registry5 and a recent randomised trial11 did not support the association of new cerebral lesions with major adverse events at follow-up. Notwithstanding this, the possible negative impact of new asymptomatic cerebral lesions at DW-MRI after CAS on cognitive function remains open to question.
Preliminary studies with routine neuropsychological tests suggested that new cerebral lesions at DW-MRI after CAS may impact negatively on cognitive function24,25, due to a microemboli-induced cerebral deterioration similar to that observed in some patients after cardiac surgery26. On the contrary, the randomised trial of Akkaya et al, which planned a routine six-month neuropsychological examination after protected CAS, failed to demonstrate a significant neurocognitive decline in patients with as compared to those without new cerebral lesions at DW-MRI11. Thus, the impact on neurocognitive function of new cerebral lesions at DW-MRI after CAS warrants further investigation.
Secondly, the present study suggests that the use of PO versus DF during protected CAS does not reduce the risk of cerebral embolisation observed at DW-MRI, neither ipsilateral nor contralateral. New ipsilateral cerebral lesions are considered a surrogate marker of device efficacy, whilst new contralateral cerebral lesions are considered a surrogate marker of device or procedure complexity, since they are mainly due to aortic arch instrumentation27. Although the risk of new cerebral lesions does not differ with PO versus DF, the present study points to a significant modification of treatment effect according to diabetes, baseline stenosis or symptoms. In the first randomised trial comparing PO versus DF for protected CAS, Montorsi et al4 divided the CAS procedure into several phases and measured the incidence of microembolic signals at transcranial Doppler echography in each phase. The highest incidence of microembolic signals associated with PO was registered during deflation of the proximal balloon, possibly due to suboptimal aspiration or wash-out of debris prolapsed through the stent struts once blood flow was restored. The highest incidence of microembolic signals associated with DF was registered while crossing the target lesion, possibly due to device/plaque attrition. Taking together previous observations and the present findings, it can be argued that the higher the grade of baseline stenosis, the greater the efficacy of PO versus DF. Indeed, PO ensures cerebral protection during CAS without crossing the culprit lesion, thus avoiding the embolisation of debris due to the mechanical disruption of the plaque. In addition, the blood-flow inversion impedes the continuous flushing of plaque components into the brain. However, the advantages associated with PO are less relevant in diabetic or symptomatic patients due to intrinsic lesion features28 that may lead to acute and subacute prolapse of plaque components even after stent implantation once cerebral protection is completely removed.
Thirdly, the present study shows that the use of PO versus DF during CAS was not associated with a lower risk of death/CVE. Continuous device and therapy iteration have transformed contemporary CAS into a highly safe and efficacious treatment strategy, through a drastic reduction of adverse events22. For this reason, comparative studies of contemporary CAS with PO versus DF for cerebral protection would require very large sample sizes to be adequately powered for rare outcomes. In the absence of such studies, the clinical results of this meta-analysis are reassuring and in line with those observed in the era of protected CAS21,22. However, the small sample size of this meta-analysis cannot definitively rule out a different clinical efficacy between PO and DF. Interestingly, in a recent meta-analysis of DW-MRI studies29, CAS with PO versus DF led to fewer new cerebral lesions/patient. However, the presence of significant statistical heterogeneity, i.e., the inclusion of studies comparing transcervical CAS with PO versus transfemoral CAS with DF (with a different inherent risk of cerebral embolisation), the lack of investigation of the observed heterogeneity and the absence of analyses addressing the clinical impact of imaging findings, reinforces the value of the current analysis.
Study limitations
The current study presents some limitations: this meta-analysis relies on data belonging to both randomised and observational studies. However, there was no interaction between study design and outcomes. The majority of the studies included were not powered for clinical outcomes, since they focused on surrogate endpoints, such as imaging-based measures of efficacy. On the one hand, the relatively small size and the selected nature of the population preclude fully exploring differences in relatively infrequent adverse clinical events. On the other hand, the lack of association between new cerebral lesions and adverse outcomes observed in our meta-analysis raises doubts about the role of such surrogate endpoints in future investigations of protected CAS. The risk estimates for cerebral embolisation were derived from studies in which patients were treated with different devices. Although efficacy profiles may vary, recent data suggest a plateau of clinical efficacy among devices used for contemporary CAS22. Almost all studies included in the present meta-analysis have a randomised design, and factors other than the type of cerebral protection during CAS are supposed to influence both treatment arms equally. In this respect, even the actual risk of embolisation in each phase of a CAS procedure cannot be derived from this study; any risk difference between groups is probably dependent on the type of cerebral protection. The observed statistical and clinical heterogeneity has been managed and thoroughly investigated, though unknown sources of heterogeneity cannot be definitively excluded. The presence of heterogeneity in the risk estimates underlines the hypothesis-generating nature of this meta-analysis, which, however, remains helpful for planning future investigations on this topic. The experience of centres in CAS (cut-off of >50 CAS/year)1 did not modify the treatment effect: the value of operator experience remains undisputed, though the minimum of CAS procedures identifying a skilled operator remains controversial30. In addition, the confidence of operators with specific devices has not routinely been reported within the included studies, and the possible influence remains unstudied. The clinical follow-up was limited to a median of 135 days. A longer follow-up would be desirable for assessing the clinical and neurocognitive impact of new cerebral lesions at DW-MRI after protected CAS. Only one trial10 among those included performed a supplemental three-month MRI after CAS: for this reason the reversibility of new cerebral lesions after CAS cannot be adequately assessed by this meta-analysis.
Conclusions
This meta-analysis suggests that one half of patients treated with CAS under cerebral protection develop new embolic cerebral lesions at DW-MRI. The vast majority of these lesions do not lead to neurologic symptoms, and their clinical impact remains to be demonstrated. Cerebral protection with PO versus DF neither reduces cerebral embolisation nor impacts on clinical outcomes. Diabetes, baseline stenosis and symptoms impact on the risk of cerebral embolisation associated with protected CAS.
| Impact on daily practice In daily practice, despite the clinical safety of contemporary protected CAS, subclinical cerebral embolisation still occurs. The possible association of these cerebral lesions with progressive neurocognitive decline remains an open question. Diabetes, baseline stenosis and symptoms increase the risk of embolisation associated with protected CAS. These features should be taken into account when selecting patients and cerebral protection systems. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
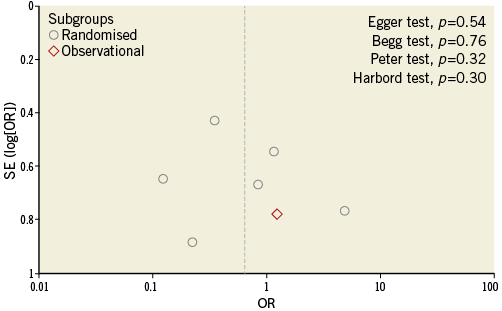
Online Figure 1. Funnel plot distribution of studies included in the meta-analysis according to primary outcome. The standard error (SE) of the logarithm of odds ratio (OR) - SE(log[OR]) - is plotted against the OR of new cerebral lesions. The absence of publication bias can be evaluated both visually and mathematically. A p-value <0.05 indicates significance.
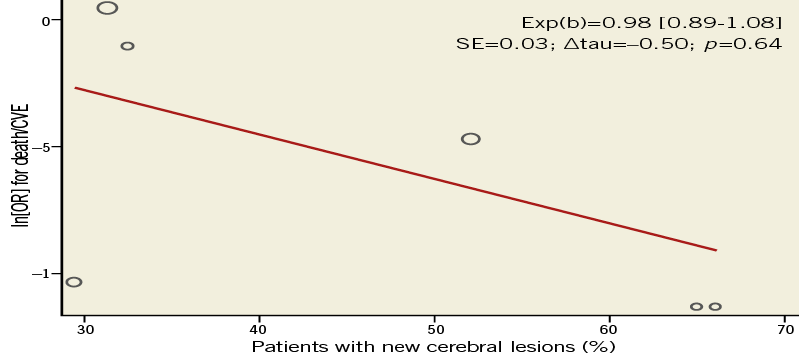
Online Figure 2. Meta-regression analysis evaluating the relationship between the incidence of new cerebral embolisation and the risk of death/cerebrovascular events (CVE). The relationship between death/CVE, measured as the natural logarithm of odds ratio - ln(OR) - for death/CVE and the incidence of new cerebral embolisation is investigated with a weighted random effect meta-regression analysis. The size of circles is proportional to the weight of each study in the fitted random-effects meta-regression. Exp(b) is presented with 95% confidence interval whilst the symbol Δ refers to “change”. A p-value <0.05 indicates significance. SE: standard error