Abstract
Aims: The aim was to determine the incidence of new ischaemic lesions on diffusion-weighted MR imaging (DWI) in a non-randomised cohort of patients after protected and unprotected carotid artery stent placement using the Parodi Anti-Emboli System (PAES).
Methods and results: A retrospective review was conducted on 269 patients who received DWI prior to, and 24-72 hours after, stent placement. All patients were enrolled in one centre. Forty patients stented with the PAES device were matched with 229 patients stented without protection (control group). New diffusion restriction on DWI was detected in 25.8% (PAES) versus 32.3% (control group); p=0.64. On average there were 0.7 lesions (PAES) versus 0.8 lesions (control group) per patient. The area of lesions was 1.7 (PAES) versus 5.6 mm2. In a subanalysis of patients (32 PAES, 148 non-protected) with >80% stenosis, the area of restricted diffusion was less when proximal protection was used (p<0.05). The number and area of DWI lesions did not differ on the contralateral, non-stented side. When the PAES system was used, patients were more likely not to have any lesion at all (p=0.028).
Conclusions: In high-grade stenosis, the use of the Gore PAES device significantly reduced the area of new DWI lesions and patients were more likely not to have any new DWI lesion at all.
Introduction
Cerebral protection devices were developed to reduce the risk of stroke caused by thromboembolic events during carotid artery stenting. The application of protection devices has been demonstrated to be feasible but there is, as yet, no level 1 evidence of benefit1-3.
Currently, three types of temporary cerebral protection are being used. These are devices with distal balloon occlusion or filtration baskets, as well as proximal occlusion devices with flow arrest or flow reversal in the internal carotid artery (ICA). However, in the large investigations in which carotid artery stenting (CAS) was compared with carotid endarterectomy (CEA)4,5 only distal protection devices were used.
The current study was undertaken to determine the incidence of new ischaemic lesions detected on diffusion-weighted MR imaging (DWI) in a non-randomised cohort of patients after protected and unprotected carotid artery stent placement using a proximal protection device, namely the Parodi Anti-Emboli System (PAES) (WL Gore & Associates Inc., Flagstaff, AZ, USA). Diffusion-weighted imaging (DWI) was used as it has the ability to visualise changes in diffusion within minutes after the onset of ischaemia, allowing it to become a powerful tool in the evaluation of patients with stroke syndrome6,7. DWI has been suggested as a surrogate outcome measure for treatment safety in future pilot studies of carotid interventions8.
Methods
Over a six-year period from 2000 to 2006, a retrospective review was conducted in our centre on 269 patients who received DWI imaging prior to, and 24-72 hours after, stent placement. Data were collected as part of an audit. All patients agreed that their data could be used for research purposes.
Patients with both symptomatic and asymptomatic stenosis were included. Symptomatic patients were defined as those with transient ischaemic attacks (TIA), or minor or major stroke in the territory of the stent-treated side within the previous four weeks. All patients were surveyed on a dedicated stroke unit after the procedure, and neurological examination was conducted by an experienced neurologist before and after the intervention and on the day of discharge. All neurological changes were recorded.
The following stents were used: WALLSTENT® (Boston Scientific, Natick, MA, USA); S.M.A.R.T.® nitinol stent (Cordis Corporation, Johnson & Johnson, Warren, NJ, USA) or Cordis PRECISE® nitinol stent system (Cordis Corporation); and Zilver vascular stent (William Cook Europe, Bjaeverskov, Denmark).
All patients received adjunctive dual antiplatelet therapy with ASA and clopidogrel. Interventionalists had a minimum experience of 25 CAS procedures. Forty patients were stented using the Gore Parodi Anti-Emboli System (PAES). Data from these patients were compared to data from 229 patients who were stented without a protection device. Patients where distal protection devices were used were excluded.
PAES was only used in patients who had a good circle of Willis with no haemodynamically relevant stenosis on the contralateral side. PAES was used at the operator’s discretion and discussed with the patient. It was not used in cases of estimated high-risk carotid disease, such as floating thrombus or ulcerated plaque.
All patients were evaluated with cerebral DW MRI prior to and after stent placement the day before, and up to 72 hours after CAS. Whole-brain diffusion-weighted images were acquired with an echo-planar sequence. An isotropic sequence was used (repetition time: 4,000 m/s; echo time: 133 m/s; field of view: 210 mm; matrix: 128×128; and number of excitations: 4), with b values of 0 and 972 s/mm2. All new hyperintensities on the brain scans were noted and categorised according to size, frequency and location. Lesions were counted and then categorised by size in mm². This was done for the vessel-dependent (stented side) and vessel-independent (contralateral) areas.
Comparison was made between preprocedural and post-procedural diffusion-weighted imaging. All new lesions on DWI which occurred after CAS were counted, and the number and area (mm²) of lesions were measured.
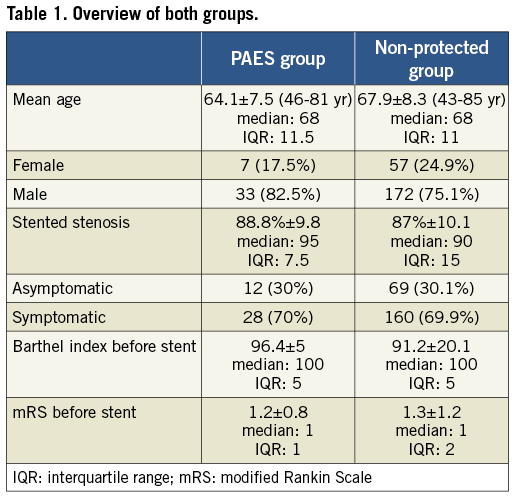
Statistical analysis was done using Statistical Product and Service Solutions (SPSS) (SPSS Inc., Chicago, IL, USA). Non-parametrical tests (chi-square test, Mann-Whitney test) were used. A significance was established at p<0.005 (Table 1).
Results
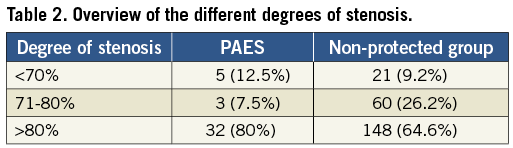
Patients were asymptomatic in 81 cases and symptomatic in 188 cases. The age range of the patients was 43-85 years, with a mean of 67.3±8.3. We had 64 female and 205 male patients (Table 1). The mean degree of stenosis (according to the North American Symptomatic Carotid Endarterectomy Trial) on the treated side was 87.3% ±10 (Table 2).
There were no discernible differences between the two groups in terms of degree or appearance of stenosis, patient age, gender, risk factors such as hypertension, smoker, former smoker, diabetes, hyperlipidaemia, stent type used and symptomatic and asymptomatic patients (p>0.05).
Two patients did not tolerate temporary occlusion of the common carotid artery (CCA). These patients were therefore stented without protection and were excluded from the study. In both of these cases, absence of new lesions was detected at post-interventional MRI.
The number of new DWI lesions that occurred on the stented side was 0.81±1.8, and the mean area (mm2) of fresh DWI lesions on the stented side was 5.0±14.3 mm2.
There was no significant difference concerning clinical outcome or fresh DWI lesions when comparing the two groups. The Barthel index score before stent placement was 91.6±19.3, and the Barthel index score after stent placement was 92.5±18.3.
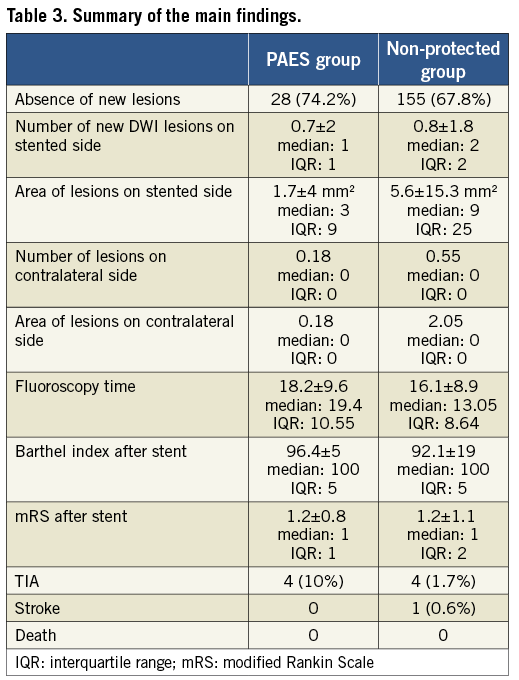
After the stent procedure 74.2% of patients in the PAES group had no new diffusion restriction on DWI. In the non-protected group only 67.8% showed absence of new lesions. However, this was not statistically significant.
The mean number of new DWI lesions was 0.7 in the PAES group vs. 0.8 in the non-protected group. Although the area of lesions was 1.7 mm2 (PAES group) versus 5.6 mm2 (non-protected group) on the stented side, this was not significant (p=0.064; Mann-Whitney U test) (Table 3, Table 4).
Subanalysis of patients with >80% stenosis
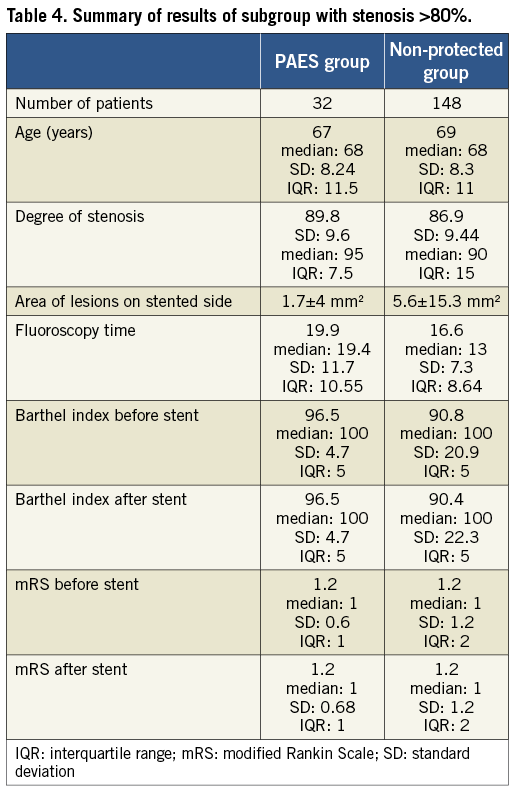
We performed a subanalysis in those patients with a >80% stenosis. The group consisted of 32 PAES patients and 148 non-protected patients. We found:
1. Total area of lesions
The overall area in mm2 (all new lesions added together) of diffusion restriction was less when proximal protection was used (p<0.034).
2. Patients with new DWI lesions (>80% stenosis)
On the contralateral, non-stented side, the number and the area of fresh DWI lesions did not differ between the two groups.
3. Patients without new DWI lesions (>80% stenosis)
When comparing patients where no fresh DWI lesions occurred after stenting we found that when the PAES system was used patients were more likely not to have any lesion at all (p=0.028).
Clinical results
There was no difference in clinical outcome after the intervention between the PAES group and the non-protected group. This included technical success rate, Barthel index and mRS score before and after intervention, as well as peri-interventional and post-interventional TIA, minor and major stroke.
Discussion
The use of distal cerebral protection devices has recently been the subject of debate in which it has been suggested that they may generate more microemboli than are demonstrated in unprotected CAS7-10.
The occurrence of DWI lesions in interventional procedures is considered a non-clinical measure of outcome. However, there are data supporting the finding that embolic events can also occur in the early post-procedural phase and that they are related to interactions between the stent frame and the plaque (poor stent scaffolding, plaque prolapse, platelet micro-aggregates, etc.)11,12. Some of the DWI spots detected in this study may thus not have been related to the use (or non-use) of a protection device.
A subgroup analysis of the International Carotid Stenting Study (ICSS) found that new DWI lesions occurred significantly less frequently when no distal protection was used (68% vs. 35%), OR 3.28 (1.50-7.20); p=0.0038.
The German Stent-Supported Percutaneous Angioplasty Carotid Artery versus Endarterectomy (SPACE) trial found no significant difference regarding the stroke/death rate in unprotected vs. protected CAS (6.2% vs. 8.3%; p=0.40)13.
Two randomised studies3,14 evaluated the use of filter protection devices in CAS compared to non-protected stenting regarding the amount of distal cerebral emboli. Both found a non-significant increase in lesions on DWI in the filter-protected group.
In a total of 30 patients, Macdonald et al3 detected significantly more transcranial Doppler (TCD) signals (426.5 vs. 165.2, p=0.01) and emboli (251.3 vs. 92, p=0.03) in procedures using filter protection than during interventions without protection. DWI at one to three hours post procedure and 24 hours after stenting showed 29% new lesions in the protected group and 18% (4 of 22) in the unprotected group (p=0.38).
The most recent and largest randomised trial, namely the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST)15,16, evaluated filter-protected CAS with carotid endarterectomy. The CREST investigators included 2,502 symptomatic and asymptomatic patients with carotid artery stenosis with (symptomatic, ≥50% stenosis detected by angiography or ≥70% stenosis detected by ultrasound) or without previous stroke (asymptomatic, ≥60% stenosis detected by angiography or ≥70% stenosis detected by ultrasound; n=1,181).
The primary endpoint of the study was defined as the 30-day rate of stroke, myocardial infarction and death combined with the rate of ipsilateral stroke over four years after randomisation. The results revealed no significant difference between the CAS and the CEA groups (7.2% vs. 6.8%; p=0.51; 95% CI) at a median follow-up of 2.5 years17. The event rate at 30 days was 5.2% in the CAS group versus 4.5% in the surgery group. The risk of stroke and death was slightly higher in CAS than in CEA (4.1% vs. 2.3; p=0.01, and 0.7% vs. 0.3%; p=0.18). It seemed that patients older than 70 years had more benefit from surgery whilst younger patients benefited more from stenting. A disadvantage of the study might be that the combination of asymptomatic patients (with a lower risk for stroke) and symptomatic patients might reduce periprocedural event rates, and could eventually disguise real differences between CAS and CEA. Long-term follow-up results showed that both approaches had comparable results and are equivalent regarding stroke prevention17.
Although distal protection devices might have been able to reduce the incidence of periprocedural complications, we cannot be certain of this. Distal protection devices first have to cross the (usually tight) carotid stenosis, thereby carrying the risk of dislodging debris. Often, predilatation of the stenosis is necessary to pass the filter or balloon device. Therefore, a system specifically protecting cerebral flow during the entire procedure seems an ideal option.
The Gore PAES proximal protection system consists of a balloon guide that is placed proximally to the stenosis, within the common carotid artery. By placing a second balloon within the external carotid artery it provides distal neuroprotection during CAS by means of continuous blood flow reversal, thereby directing emboli away from the brain. The procedure has been described elsewhere18, and there is robust evidence regarding the safety and feasibility of the device19.
Although the clinical relevance of silent microembolic events has not yet been established, an association with cognitive impairment20 has been suggested. Purandare et al21 discussed cerebral emboli as a potential cause of Alzheimer’s disease and vascular dementia.
In the ICSS study, the median number of DWI lesions after intervention was three (IQR 1-9)8. In our single-centre experience the number of new DWI lesions that occurred on the stented side was 0.81±1.8.
In the largest series of DWI controlled, unprotected carotid artery stent procedures so far, in 197 patients7, new DWI lesions occurred in 29.9% in the stented brain territory.
Although in our patient group we could not establish a significant difference regarding the number of new DWI lesions between the PAES and unprotected group (25.8 vs. 32.3; p= 0.64), the area of lesions was significantly smaller when a proximal protection device was used (p<0.05). Former studies that only considered the number of lesions after CAS would have missed this important fact.
Lesions on diffusion-weighted magnetic resonance imaging are not necessarily associated with immediate neurological sequelae and often remain clinically silent. As expected, our clinical results with and without protection did not differ concerning morbidity and mortality, TIA and prolonged reversible ischaemic neurologic deficit (PRIND). The incidence of clinically symptomatic stroke during carotid artery stenting is so low that several thousand patients would be necessary to demonstrate significant differences between groups. DWI serves as a surrogate marker to analyse the differences between protected and unprotected CAS in this small number of patients.
Our results showed that, when the PAES system was used, patients were more likely not to have any lesion at all (p=0.028). This is in accordance with previous results.
In a previously published evaluation of flow reversal in dogs, complete protection against embolic debris could be achieved22. Parodi et al23 also described complete absence of microemboli with flow reversal, whereas alternative methods were unable to achieve this level of protection. Rabe et al24 also demonstrated complete absence of new DWI lesions following CAS with flow reversal.
Montorsi et al25 conducted the first randomised study and compared the rate of cerebral microembolisation during CAS with proximal versus distal cerebral protection. Fifty-three patients were randomised to undergo CAS with proximal protection (Mo.Ma® Ultra proximal cerebral protection device; Medtronic/Invatec, Roncadelle, Italy) or distal filter protection (FilterWire EZ™ Embolic Protection System; Boston Scientific). The authors assessed microembolic signals (MES) using TCD and lesions on diffusion-weighted MRI. In patients with high-risk, lipid-rich plaque, Mo.Ma® significantly reduced MES counts during lesion crossing, stent crossing, stent deployment, stent dilatation and in total. No significant difference was found in the number of patients with new post-CAS embolic lesions. Another study (PROFI)26 compared embolic lesion load as seen on DWI between filter-protected and proximal balloon-protected CAS in a randomised trial. Compared with filter protection, proximal balloon occlusion resulted in a significant reduction in the incidence of new cerebral ischaemic lesions. Also, the number and the volume of new cerebral ischaemic lesions were significantly reduced by proximal balloon occlusion.
A recent European, prospective, multicentre study conducted in 2012 investigated the outcome of patients who underwent CAS for asymptomatic and symptomatic carotid artery stenosis (n=122; 28% symptomatic) using the GORE flow reversal system27. The primary and secondary endpoints of this study were stroke/death rate and myocardial infarction or a non-stroke-related neurological event within 30 days post intervention.
In three of the 122 patients the device could not be used, due to vessel tortuosity in one case and intolerance in two cases. No death or MI occurred during the 30-day period. The major adverse events rate at 30 days and the secondary endpoint rate were both 1.6%. A transient ischaemic attack was seen in one patient of each study arm.
Despite these and our positive results, a definitive identification of any substantial differences with the Gore PAES systems in carotid artery stenting will not be possible until a randomised trial has been conducted.
Conclusion
Use of the Gore PAES device significantly reduced the area of new DWI lesions in patients with high-grade (>80%) stenosis. In addition, when the flow reversal system was used, patients were more likely not to have any DWI lesions at all. To establish the efficacy of proximal protection devices, a randomised comparison of non-protected versus filter-protected carotid artery stenting is warranted.
Conflict of interest statement
I.Q. Grunwald, W. Reith and P. Papanagiotou have previously received consulting fees, travel expenses, congress support or study honoraria from Boston Scientific, Bard, ev3, Gore, Guidant, and Cordis. The other authors have no conflicts of interest to declare.

