Abstract
Aims: To assess the use of proximal protection devices in consecutive patients as the preferred means of cerebral embolic protection for primary carotid stenting.
Methods and results: This was a prospective single-centre study to evaluate the technical and clinical success of proximal protection devices as the first choice for embolic protection in symptomatic (≥50%) and asymptomatic (≥70%) carotid stenosis. Proximal protection devices were used for embolic protection in 124 consecutive patients. No patients were excluded for anatomical reasons. The GORE® Flow Reversal System (W.L. Gore, Flagstaff, AZ, USA) was used in 92 patients, and the Mo.Ma Ultra device (Medtronic, Minneapolis, MN, USA) in 32 patients. Follow-up duration was 30 days. Mean age was 71±8 years. Seventy-five percent of patients were male (n=93). Twenty-six of 124 (21%) treated stenoses were symptomatic. Technical success was achieved in 122 of 124 cases (98%). Due to anatomical conditions, in two patients flow reversal could not be established. In both cases additional distal filter devices were used. Carotid stenting was successful in 124 lesions (100%). Ten patients (8.1%) had contraindications to flow reversal (three high-grade ostial stenoses of the external carotid artery, seven contralateral occlusions of the internal carotid artery) in none of whom complications occurred. There were no procedural neurologic events. Within 30 days of follow-up, one patient had an ischaemic stroke (on day 11).
Conclusions: Proximal protection is a safe method as the first choice for embolic protection. It can be used with a high rate of technical success.
Introduction
Carotid stenting has been used with success to prevent strokes1,2. Similar to carotid endarterectomy, the procedure itself is associated with a risk of stroke, partially offsetting its benefit. Distal protection devices are used to minimise the procedural stroke risk. However, embolic events occur despite the use of these devices3. While all available protection systems are capable of capturing macroemboli, the distally placed filter systems may in fact increase the rate of microembolisation, as demonstrated during procedural transcranial Doppler (TCD)4,5. The GORE® Flow Reversal System (W.L. Gore & Associates, Inc., Flagstaff, AZ, USA) and the Mo.Ma Ultra (Medtronic, Minneapolis, MN, USA) were developed as proximal protection devices to provide embolic protection prior to crossing the lesion in order to minimise the possibility of adverse neurologic events. Though data demonstrating low periprocedural stroke rates using proximal protection have been published6, a strategy of using proximal protection as the dedicated first choice in consecutive (unselected) patients has not been explored. Therefore, this study assessed the use of proximal protection devices as the dedicated first choice for embolic protection in carotid artery stenting.
Methods
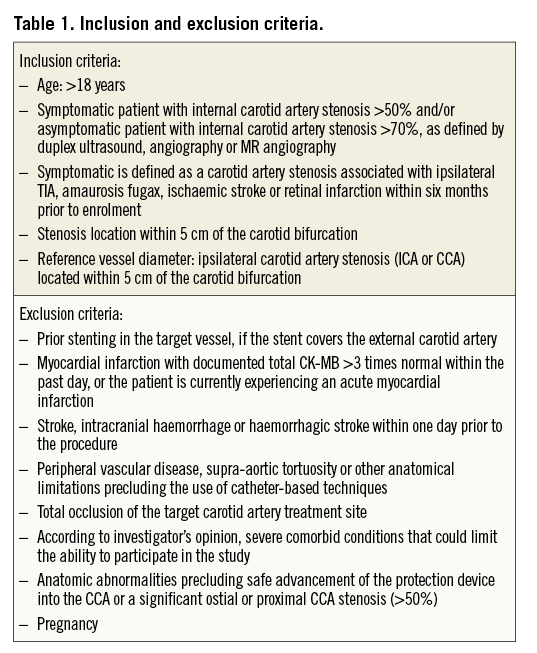
This is a prospective, non-randomised, single-centre independent evaluation enrolling consecutively symptomatic patients with angiographically proven diameter stenosis >50% or asymptomatic patients with diameter stenosis >70% in the proximal internal carotid artery or at the carotid bifurcation. The detailed inclusion and exclusion criteria are outlined in Table 1.
The following tests were performed prior to the procedure to verify the eligibility criteria: a physical examination (heart rate and blood pressure) was performed and relevant medical history including cardiovascular risk factors, past cardiovascular events (history of myocardial infarction, stroke, revascularisation), current symptoms of concomitant cardiovascular disease, medication and patient demographic information was documented. Colour-coded Duplex ultrasound assessment of the extracranial vessels or magnetic resonance (MR) angiography or selective angiography, routine laboratory tests (including cardiac enzymes) and a 12-lead electrocardiogram were performed.
MEDICATIONS
All patients received 100 mg of aspirin orally within 24 hours of the procedure and 75 mg of clopidogrel daily at least three days before the intervention or a loading dose of 300 mg of clopidogrel the day before the procedure. During the procedure, all patients received intravenous heparin (5,000 to 10,000 IU) to maintain an activated clotting time (ACT) of at least 250 seconds. Measurement of the ACT was repeated and documented every 30 minutes throughout the procedure. To avoid significant bradyarrhythmias during predilatation and stent post-dilatation, 1 mg of atropine was administered two to three minutes before dilatation. Post procedure, all patients received at least 100 mg of aspirin daily indefinitely and 75 mg of clopidogrel daily for a minimum of 30 days.
NEUROLOGICAL ASSESSMENT
A complete neurological examination and NIH (National Institute of Health) Stroke Scale evaluation was performed by a certified investigator within seven days prior to the procedure, at discharge and at 30 days if neurological symptoms occurred. Neurological events were categorised as transient ischaemic attacks (TIA), minor strokes and major strokes. In cases of suspected neurological events, cranial imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) was performed.
ANGIOGRAPHY
Angiography and stenting were performed using local anaesthesia at the vascular access site. Baseline angiographic imaging of the ipsilateral side included at least two projections to determine subject eligibility. Imaging of the ipsilateral intracerebral circulation was performed immediately pre- and post-procedure. Angiography of the contralateral side was not performed routinely. Angiography during balloon inflation was used to verify that flow reversal had been established.
PROCEDURAL REQUIREMENTS
Electrocardiogram, heart rate and blood pressure were continuously monitored throughout the procedure. In addition, periodic neurologic assessments were performed during every critical step of the procedure. In all patients a proximal protection device was used. We started our investigation with the GORE Flow Reversal System. During the course of the study we had to switch to the Mo.Ma Ultra because the GORE Flow Reversal System was not available anymore. However, we used the Mo.Ma Ultra in the same way, i.e., with flow reversal. The use of both devices has previously been described in detail elsewhere7-10. Briefly, after standard angiographic evaluation of the stenosis, the balloon sheath is placed in the common carotid artery over a 0.035-inch guidewire, and the balloon wire with the occlusive balloon is positioned in the external carotid artery. Subsequently, using the GORE Flow Reversal System, the proximal hub of the balloon sheath was connected to a sheath in the femoral vein via an interposed filter system. Before the target lesion was crossed with a guidewire, both the proximal and distal balloons were inflated at low pressure, thereby blocking blood flow towards the brain. Using the GORE Flow Reversal System, the difference between the arterial pressure in the carotid artery and the venous pressure in the femoral vein results in reversal of blood flow in the carotid artery, thereby creating a shunt that directs emboli away from the brain and towards the venous system. Using the Mo.Ma Ultra we created flow reversal by leaving the stopcock of the working channel open to create a pressure gradient between the arterial pressure in the target vessel and the air pressure in the catheterisation laboratory. Once flow reversal was established, carotid stenting was performed using commercially available stent systems.
POST-PROCEDURE/DISCHARGE EVALUATION
Cardiac enzymes were drawn six to 12 hours after the procedure and, if values were above the normal limit, a second draw was needed prior to discharge. Neurological assessment (NIH Stroke Scale evaluation) was performed before discharge.
30-day follow-up visit
Neurological assessment was required at 30 days post procedure if a patient reported any neurological symptoms. A cardiovascular history was performed and medications were documented.
The primary endpoint was any major adverse event within 30 days after carotid intervention, with major adverse event defined as any new neurological event: TIA, minor or major stroke. A TIA was defined as an acute neurological deficit lasting less than 24 hours, of presumed vascular origin, confined to an area of the brain or eye perfused by a specific artery, and without evidence of a new ischaemic brain infarct upon brain imaging. Importantly, transient non-specific neurological symptoms limited exclusively to the duration of flow interruption by balloon occlusion with immediate recovery after re-establishment of antegrade flow were not considered transient ischaemic attacks. The criteria for strokes were new neurological deficits with new ischaemic or haemorrhagic defects on CT or MRI. A minor stroke was defined as any new neurological disorder with an NIH Stroke Scale score of less than five points or, if the NIH Stroke Scale score was more than four points at enrolment, any increase of less than five points. A major stroke was defined as a new neurologic disorder with an NIH Stroke Scale score of five or more points or, if the NIH Stroke Scale score at enrolment was more than four points, any increase of five or more points.
The secondary endpoint was a composite of technical and clinical success. Technical success was achieved when the protection device was delivered, placed, reverse flow established and the balloon sheath and the balloon wire retrieved, as outlined in the instructions for use of each device, without causing any adverse event. Clinical success was evaluated 24 to 48 hours post procedure, and was defined as application of a proximal protection device in the absence of death or stroke.
The study was approved by the local ethical committee, and all patients gave written informed consent prior to the procedure.
Statistical analysis was performed on an intention-to-treat principle. Nominal and categorical variables are displayed as frequencies and percentages. Values for continuous variables are expressed as means plus standard deviation. All data were analysed using BiAS for Windows™ (Version 10.04, © 1989-2013; epsilon-Verlag, Darmstadt, Germany).
Results
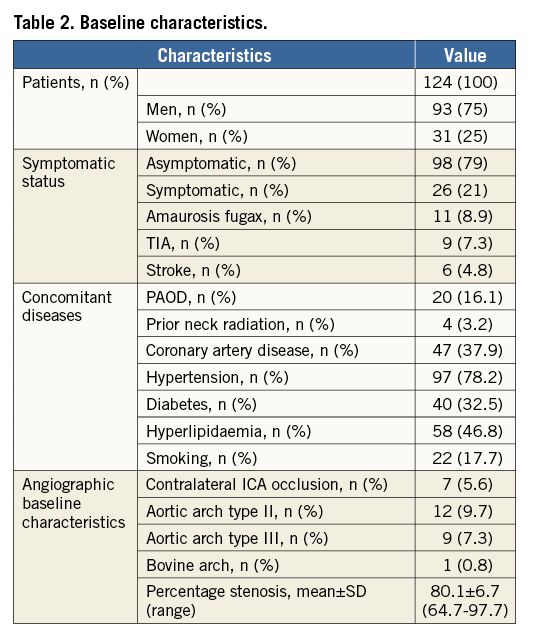
A total of 124 consecutive patients matching the inclusion and exclusion criteria were enrolled (Table 1). Mean patient age was 71.9±8.5 years (range 55 to 94 years) and 75% (n=93) were male. Twenty-six patients (21%) had a symptomatic carotid stenosis. Six patients (4.8%) had a previous stroke and 20 patients had symptoms that lasted less than 24 hours: nine with transient ischaemic attacks (TIA; 7.3%) and 11 with amaurosis fugax (8.9%). Mean time between the neurological index event and the carotid intervention was 50.5 days with a range of two to 181 days in symptomatic patients. Baseline patient characteristics are outlined in Table 2.
The mean diameter stenosis was 80.1±6.7% (range 64.7 to 97.7%) at baseline angiography without significant difference between symptomatic (79.5±6.4%) and asymptomatic patients (80.6%±6.7%). An occlusion of the contralateral internal carotid artery was found in seven patients (5.6%), and the distribution of aortic arch types was: 82.3% Type I (n=102), 9.7% Type II (n=12), 7.3% Type III (n=9), with 0.8% bovine (n=1).
The average procedure time was 61.2±25.9 minutes with an average fluoroscopy time of 13.9±8.6 minutes. The mean time of endovascular carotid flow occlusion was 11.4±6.1 minutes (range two to 37 minutes).
Predilatation before crossing the lesion was performed in 41 of 124 stenoses (33.1%). One hundred and twenty-five stents were implanted in 121 patients. A second stent was implanted within the first in four patients due to severe plaque protrusion after implantation of the first stent. The distribution of implanted stent systems is shown in Table 3. In the majority of cases, stents with an open cell design were selected (n=84; 67.7%).
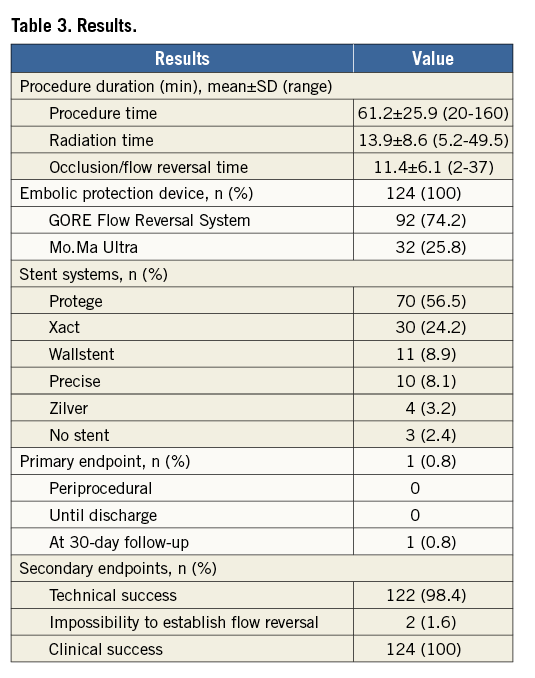
In the three remaining patients, there was no need for the implantation of a stent. They had restenosis after stent implantation treated successfully with balloon angioplasty.
The primary endpoint (TIA, minor or major stroke during the procedure or within 30 days of follow-up) occurred in one patient (0.8%), a 74-year-old male with an asymptomatic stenosis of the right internal carotid artery. After successful implantation of a Protege® stent (ev3 Endovascular Inc., Plymouth, MN, USA) under embolic protection using a Mo.Ma Ultra device, the patient was discharged without neurological symptoms two days after the intervention. Nine days after discharge, he was admitted to a local stroke unit with a major stroke due to thrombotic stent occlusion.
The secondary endpoint (composite of clinical and technical success) was achieved as follows: clinical success was reached in all 124 patients, and technical success in 122 of 124 interventions (98.4%). All interventions were successfully completed without intraprocedural complications. Temporary procedure-related neurological symptoms due to intolerance of interruption of antegrade flow during balloon occlusion were observed in ten patients (8.1%). Importantly, these symptoms were limited to the duration of balloon occlusion with prompt and complete recovery once antegrade flow was re-established.
In two cases (1.6%) the secondary endpoint for technical success was missed. Both of these cases were related to the use of the GORE Flow Reversal System. In five patients it was not possible to occlude the superior thyroid artery using the external carotid artery balloon. After inflation of the occlusion balloon in the common carotid artery and a test injection of contrast medium, despite the inability to occlude the superior thyroid artery in three patients, a flow reversal was established and stenting performed without further complications. However, in two patients the contrast injection demonstrated that antegrade flow to the brain remained. Therefore, a second (distal) embolic protection device was necessary. In both cases a distal filter device (GORE Embolic Filter; W.L. Gore & Associates, Inc.) was introduced through the GORE balloon sheath into the target vessel. Thereafter, the intervention was successfully completed without complications.
In five cases there were minor technical problems that could be resolved easily, which therefore did not meet the criteria for the secondary endpoint. In two subjects, we observed that there were repeated spontaneous deflations of the external carotid artery occlusion balloon. It needed several re-inflations until a stabile reverse flow could be established. In two other patients, after successful stent implantation and deflation of both occlusion balloons, the balloon positioned in the external carotid artery could not be removed. In both subjects, we inflated and deflated the balloon one more time allowing successful removal.
In one patient, after successful implementation of a flow reversal, during advancement of the stent system the external carotid occlusion balloon migrated slightly, allowing temporary antegrade blood flow. We removed the stent system, advanced a distal filter device via the balloon sheath (SpiderFX; ev3 Endovascular Inc.) and positioned it distal to the target lesion. Thereafter, we repositioned the balloon wire in the external carotid artery and established a reverse flow by balloon inflation. A 9×20 mm Protege stent (ev3 Endovascular Inc.) was deployed successfully.
None of the patients in whom the use of a proximal protection system was technically unsuccessful or was associated with technical problems suffered from neurological or other complications.
Discussion
This case series shows that proximal embolic protection devices can be used as the first choice for embolic protection during carotid stenting with high rates of technical and clinical success as well as a very low incidence of cerebral ischaemic events. In fact, no procedural strokes or TIAs occurred. Though a low periprocedural event rate has been demonstrated in prior studies examining proximal protection8-11, in these studies it was not the dedicated first choice used in consecutive patients. This raises the possibility of selection bias. Importantly, to minimise potential selection bias, proximal protection was used as the dedicated first strategy in all consecutive patients undergoing carotid stenting. Furthermore, the results of our study are comparable to a recently published meta-analysis by Bersin et al6, demonstrating that categorisation of patients into risk groups for carotid interventions may not apply to carotid stenting using proximal embolic protection devices. Studies examining carotid stenting using distal embolic protection systems have identified age (older than 80 years) and symptomatic status as risk factors of major adverse events1,12-14. We included 25 octogenarians (20.2% of the sample size) in our series, and the target lesions of 26 patients were symptomatic. None of these patients experienced complications within 30 days of follow-up. Likewise, in the meta-analysis by Bersin et al, though the incidence of periprocedural strokes was slightly higher in octogenarians, it remained low at 2.4%6. Furthermore, symptomatic status had no impact on the stroke risk. The incidence of neurologic complications reported in the aforementioned meta-analysis (stroke risk of 1.7% at 30 days) and our study (0.8% at 30 days) is substantially lower than reported in most trials comparing carotid stenting using distal protection devices to carotid endarterectomy. For example, the 30-day stroke risk was 2.1% in ARCHeR11, 3.1% in the actual treatment analysis in SAPPHIRE2, 4.1% in CREST1, and 8.6% in the protected group in EVA-3S15. These observations suggest a more reliable prevention of periprocedural neurologic events with proximal compared to distal protection. The reasons are speculative but include lesion manipulation only after embolic protection and more complete protection with flow reversal similar to vascular clamping during carotid endarterectomy. This has important implications when comparing the safety and efficacy of carotid stenting to carotid endarterectomy, and suggests that the merit of carotid stenting when compared with carotid endarterectomy should be re-evaluated using proximal protection.
Previously published trials on the use of the GORE Flow Reversal System and the Mo.Ma Ultra device have shown an average proximal occlusion time of three to 15 minutes1,8-10,16. During this time, the contralateral and posterior cerebral circulation (via the circle of Willis) allows sufficient perfusion to avoid prolonged cerebral ischaemia. Provided the posterior circulation is adequate, even an occlusion of the contralateral internal carotid artery can be well tolerated by most of the patients. In our patient series seven subjects had a contralateral occlusion. None of these patients had any symptoms of intolerance during balloon occlusion. If symptoms do occur during flow reversal or proximal occlusion they can usually be controlled by blood pressure management or intermittent balloon deflation to restore antegrade blood flow. For these cases, a stepwise approach with inflation for certain steps of the procedure allowing antegrade perfusion between steps may minimise ischaemic time in patients with an insufficient cerebral collateralisation. Data suggest that most endovascular clamping intolerances can be avoided by careful monitoring of the proximal occlusion pressure9,17. An occlusion pressure of less than 40 mmHg after inflation of the common carotid occlusion balloon seems to be the best predictor of intolerance during balloon inflation.
Limitations
All procedures were performed by an experienced single operator. Therefore, our results may not be completely transferable to other centres. Due to the very small number of neurological complications we cannot make a statement regarding risk factors for clinically apparent embolic events. Most importantly, this study has a non-randomised single-arm design with unblinded investigators. Hence, patient selection and operator bias cannot be excluded, and definitive statements regarding the merit of carotid stenting with proximal protection when compared to distal protection or endarterectomy cannot be made.
Conclusion
The use of proximal embolic protection devices as first choice for embolic protection in carotid stenting procedures is safe and feasible. The rate of neurological complications is below the requirements of the American Heart Association/American Stroke Association18 for carotid stenting procedures in asymptomatic patients (<3%) as well as in symptomatic patients (<6%).
| Impact on daily practice The crux of endovascular treatment of carotid artery stenoses is partial insufficiency of distal embolic protection devices. The proximal protection devices were developed to provide embolic protection prior to crossing the lesion in order to minimise the possibility of adverse neurologic events. This study shows that proximal embolic protection is a safe method as the first choice for embolic protection, and can be used with a high rate of procedural success. |
Conflict of interest statement
H. Sievert reports having ownership interest in CardioKinetix, Access Closure, Velocimed, Lumen Biomedical, Coherex and SMT, receiving grant research support from Cook and St. Jude Medical, and serving as a consultant for Abbott, Access Closure, AGA, Angiomed, Aptus, Atrium, Avinger, Bard, Boston Scientific, BridgePoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, Covidien, CSI, CVRx, EndoCross, ev3, FlowCardia, Gardia, Gore, Guided Delivery Systems, InSeal Medical, Lumen Biomedical, HLT, Lifetech, Lutonix, Maya Medical, Medtronic, NDC, Occlutech, Osprey, Ostial, PendraCare, pfm Medical, Recor, ResMed, Rox Medical, SentreHeart, Spectranetics, SquareOne, Svelte Medical Systems, Trireme, Trivascular, Venus Medical, Veryan and Vessix. The other authors have no conflicts of interest to declare.

