Abstract
Aims: Carotid artery stenting (CAS) has become an alternative to carotid endarterectomy in the treatment of carotid artery disease. The use of an embolic protection device (EPD) can reduce the frequency of embolic events during CAS. Difficult vascular anatomy may complicate current generation EPD placement. This problem is addressed by a new EPD, the GARDEX System. The aim of this study was to assess the safety and performance of the GARDEX EPD during CAS.
Methods and results: Thirty-eight patients underwent CAS with the GARDEX EPD in two medical centres. All patients were prospectively followed up for 30 days. Device performance and procedural details were collected and analysed prospectively. Vessel anatomy and lesion morphology were evaluated and stratified into a scoring system for anatomic difficulty. More than a third of the patients were considered to have difficult vascular anatomy for CAS. All enrolled patients were successfully treated. There was one (2.6%) minor periprocedural stroke and there were two (5.3%) periprocedural TIAs which resolved within 24 hours. No additional complications were noted during the 30-day follow-up period.
Conclusions: In this first experience, CAS under cerebral protection with the GARDEX EPD was safe and feasible. Our data suggest that the use of the GARDEX EPD is simple and shows high success rates even in challenging anatomies. The role of this new device in CAS needs to be further confirmed in a larger patient population.
Introduction
Several randomised trials have compared CAS and carotid endarterectomy (CEA) as strategies to reduce the risk of stroke in patients with significant carotid artery stenosis1,2.
The most important acute complication of carotid angioplasty is related to the distal embolisation of particles generated during the endovascular procedure with the resulting risk of stroke3. The wider carotid stenting community is convinced of the benefit of embolic protection during CAS, despite the scarcity of level 1 supporting evidence4. Current guidelines on carotid stenting state that the use of EPDs can be beneficial in reducing the risk of stroke5. The most commonly used EPDs are distal filter devices. Most of these systems are “fixed wire” systems. As a result, hostile vascular anatomy or lesion properties can make it difficult to advance the filter across the lesion with existing filter systems6. A new filter device, the GARDEX™ EPD (Gardia Medical Ltd., Caesarea, Israel), is designed as a premounted filter on a monorail catheter to be tracked over any 0.014” wire. The GARDEX System provides the physician with the ability first to cross the lesion with a 0.014” wire of choice among the broader selection of wires and then to load the GARDEX System over the wire and to deploy the filter on the guidewire, anywhere in the vessel. This report reviews data from the first 38 patients receiving CAS under cerebral protection with the GARDEX embolic protection device.
Methods
STUDY DESIGN, PATIENT SELECTION AND DATA ANALYSIS
Between December 2008 and September 2010, 38 patients underwent CAS procedures with neuroprotection using the GARDEX EPD in two medical centres (Centre for Vascular Medicine, Park Hospital Leipzig, Leipzig, Germany, and GVM Care and Research, Interventional Cardio-Vascular Unit, Cotignola, Italy). The first institution enrolled 20 patients in a first-in-man study. The protocol was approved by the local ethics committee and written informed consent was obtained from all patients. When CE mark was approved, the two institutions enrolled a further 18 patients. Both symptomatic and asymptomatic patients were treated. The degree of internal carotid artery (ICA) stenosis was measured by duplex ultrasound and was at least 50% in the symptomatic patients; in asymptomatic patients a lumen narrowing of at least 70% was considered as relevant.
All angiographic images from patients enrolled in the German institution (n=28) were retrospectively evaluated for the following anatomical properties by a single investigator (M.W.): type of aortic arch; presence of severe arch atheroma, defined as arch calcification visible on fluoroscopy; presence of bovine arch; CCA tortuosity, defined as vessel kinking of more than 60 degrees; CCA disease; ECA disease; ICA and CCA tortuosity, defined as vessel kinking of more than 60 degrees; lesion calcification and degree of stenosis, contralateral ICA disease and presence of vertebral artery stenosis. The patients were then stratified into a scoring system of four levels of vascular anatomic difficulty, as proposed by a multi-expert panel7. The following anatomic features influence the level of difficulty of a CAS procedure: tortuous CCA, great vessel origin disease, angulated distal ICA and angulated ICA origin, circumferential calcification of the lesion, diseased CCA, type III arch and bovine arch.
All patients underwent duplex ultrasound examination before and after the intervention and 30 days after the intervention. All patients underwent clinical and neurological examination before and after the procedure (within 24 hours and 30 days post intervention) by an independent observer using the National Institute of Health Stroke Scale (NIHSS). During the 30 days post intervention, all adverse events were recorded, whether or not the investigator believed them to be related to the study device. The primary endpoint was the occurrence of stroke, TIA or death during the 30-day follow-up. The secondary endpoint was the occurrence of adverse events related to the procedure or to the study device. Statistical analysis was carried out using SPSS Statistics 20 (IBM, Chicago, IL, USA). Categorical variables were presented as contingency tables with frequencies and percentage. Continuous variables were reported as the mean with standard deviation.
THE GARDEX EMBOLIC PROTECTION SYSTEM
The GARDEX System comprises a unique monorail delivery catheter that houses a pre-folded filter unit (a nitinol frame with a 120 µm pore membrane) in a “stent-like” device and a retrieval catheter to retract and collect the filter and its contents once the therapeutic procedure has been completed. Important device properties and the comparison to other currently available filter devices are presented in Table 1.
The GARDEX Embolic Protection System allows the physician to lock its stand-alone filter unit onto any standard 0.014” guidewire, anywhere along the wire downstream from the stenosis.
The GARDEX retrieval catheter uniquely introduces a retractable “nose cone” tip that overcomes the risk of stent entanglement. The monorail retrieval catheter nose cone narrows down at its tip to 0.014” in diameter in order to optimise crossing of the stented area. Once at the filter site, the nose cone tip is retracted into the catheter, allowing the collecting sheath to retrieve the filter.
TECHNIQUE OF INTERVENTION AND PERIPROCEDURAL MANAGEMENT
Consecutive patients referred for carotid artery stenting were recruited. No patient was excluded based on vessel anatomy or lesion morphology. Patients were treated with 100 mg acetylsalicylic acid and received a loading dose of 300 mg clopidogrel, if they were not already taking clopidogrel for other reasons. A daily dose of 100 mg acetylsalicylic acid and 75 mg clopidogrel was given for four weeks. Thereafter only aspirin was given. For anticoagulation, 100 U/kg of sodium heparin was given during the procedure. Diagnostic angiography and the intervention were performed via the femoral approach. After placement of a guide catheter or long sheath into the common carotid artery (CCA), a 0.014” guidewire of choice was navigated across the lesion. The following guidewires were used: Galeo ES (Biotronik SE & Co. KG, Berlin, Germany; n=21, 55.3%), ChoICE® PT Floppy Guide Wire (Boston Scientific, Natick, MA, USA; n=9, 23.7%), Hi-Torque Balance Middleweight Universal Guide Wire (Abbott Laboratories, Abbott Park, IL, USA; n=8, 21.1%). The GARDEX™ RX delivery catheter was advanced over the wire, through the lesion and the filter was locked and deployed in a location determined by the physician, two to three centimetres distal to the lesion. Predilatation, stenting and post-dilatation were performed according to the physician’s discretion. The following stents were used: Cristallo Ideale Carotid Self-Expanding Stent System (Invatec, Roncadelle, Italy; n=12, 31.6%), Precise® Pro RX® Carotid Stent System (Cordis Corporation, Bridgewater, NJ, USA; n=9, 23.7%), Carotid Wallstent® Monorail® Endoprosthesis (Boston Scientific,; n=7, 18.4%), RX Acculink Carotid Stent System (Abbott Laboratories, Abbott Park, IL, USA; n=6, 15.8%), Adapt™ Monorail™ Carotid Stent System (Boston Scientific; n=3, 7.9%), Protégé® RX Carotid Stent System (ev3 Endovascular, Inc., Plymouth, MN, USA; n=1, 2.6%). The culprit lesion was visualised in at least two different projections pre and post procedure.
Results
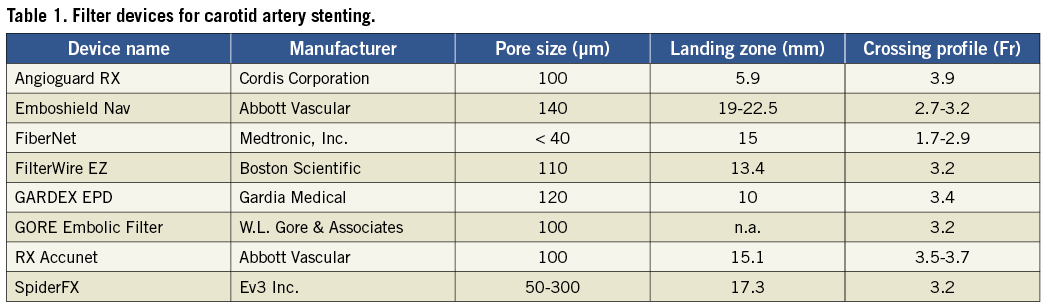
Patient and lesion characteristics are listed in Table 2. Vascular anatomic properties are presented in Table 3. The dataset was correlated to the scoring system for anatomic suitability7: 10 patients (35.7%) were considered to have very difficult vascular anatomy for CAS (Table 3). Procedural success was achieved in all subjects (100%). The mean pre-treatment degree of ICA stenosis was 81.8% (±7.7). The mean post-interventional degree of stenosis was 4.2% (±6.8) and vessel patency with a residual stenosis of less than 20% was achieved in all cases. Figure 1 shows the fluoroscopic images of a CAS procedure with the GARDEX EPD in a patient with a high grade stenosis of the left ICA. Figure 2 illustrates the GARDEX EPD ex vivo.
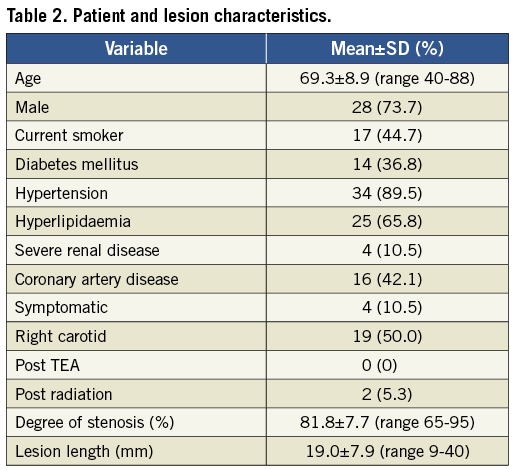
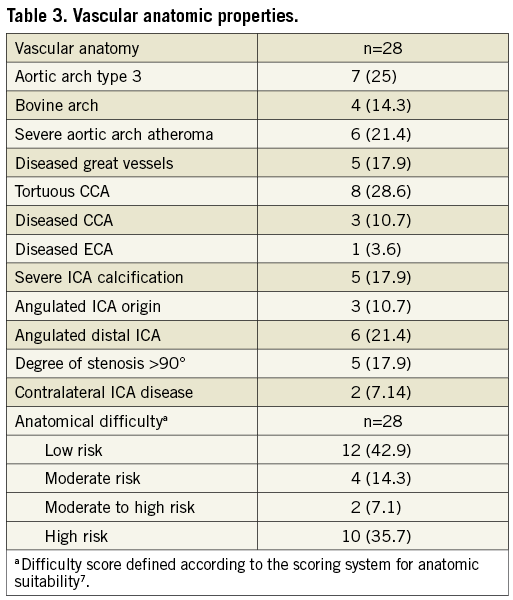
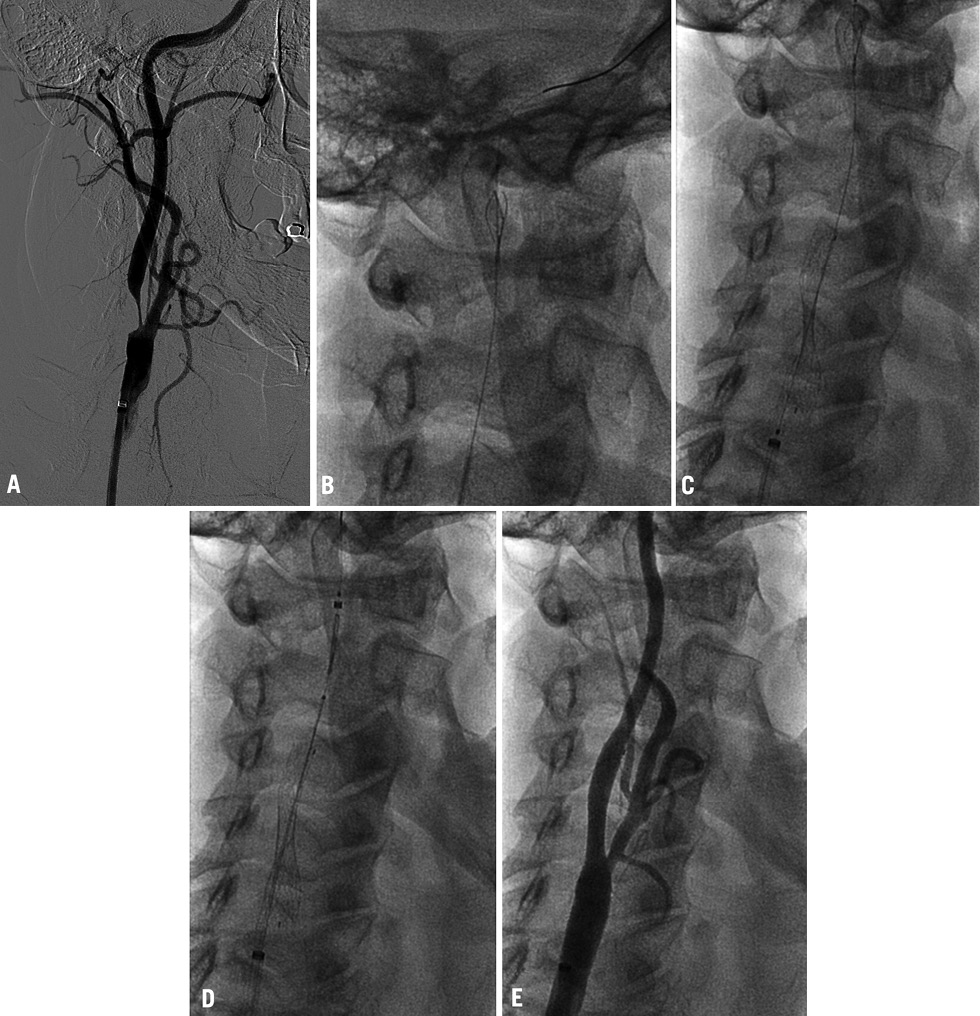
Figure 1. A) High-grade stenosis of the right ICA; B) GARDEX EPD was delivered and then deployed on the guidewire (Galeo ES guidewire) in a straight section of the right ICA; C) after predilatation a self-expanding nitinol stent (Cristallo Ideale) is deployed; D) after post-dilatation the GARDEX EPD is retrieved; E) no residual stenosis after CAS.
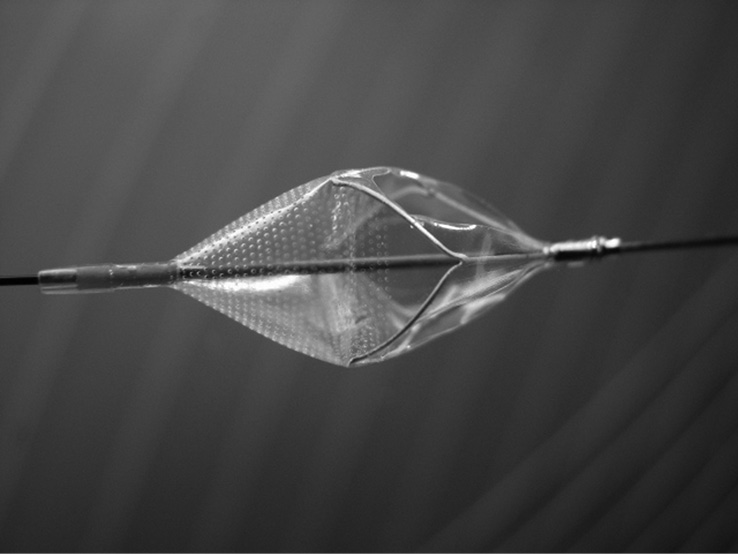
Figure 2. Close-up view of the GARDEX EPD.
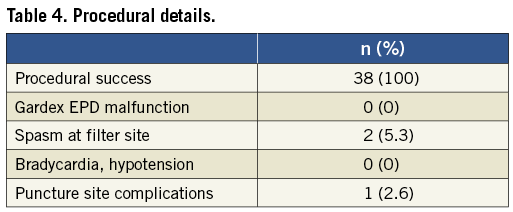
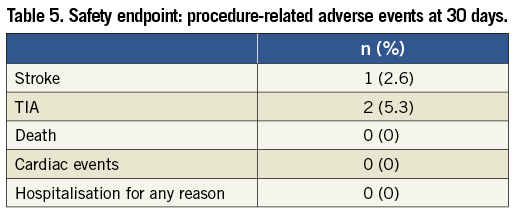
The GARDEX EPD was successfully delivered, locked and deployed in all patients. Device performance was highly graded and no device malfunctions occurred. Filter removal was performed with ease and without complications in all patients. Spasm of the ICA at the filter site was evident in two cases (6.1%). In both cases, spasm disappeared after removal of the filter. No neurological symptoms evolved in these two cases. Total flow obstruction was not observed in any case. Bradycardia and hypotension during the intervention occurred in one patient (2.6%) (Table 4). There was one (2.6%) periprocedural stroke and there were two (5.3%) periprocedural TIAs which resolved within 24 hours, all in asymptomatic patients. One puncture site complication was recorded (2.6%, bleeding without need for blood transfusion). The 30-day stroke rate was 2.6% (one minor stroke, no major stroke). No deaths, cardiac events or hospitalisations for other reasons occurred during the follow-up (Table 5).
Discussion
CAS has become a treatment alternative for patients with carotid artery stenosis. Data published so far suggest that CAS has the same efficacy as CEA in terms of long-term stroke prevention, but is associated with a higher periprocedural minor stroke rate1,8,9.
Although there is no direct comparison of protected vs. unprotected CAS, several literature reviews have indicated that EPDs have the potential to reduce the incidence of plaque embolisation during CAS10,11. Currently, filter devices are the most commonly used EPDs; however, they have several potential disadvantages: embolisation of particles smaller than the pore size of the device, possible embolisation during lesion crossing or device retrieval, difficulty in navigating severely stenosed or tortuous vessels, potential for spasm or dissection of the ICA and incorrect wall apposition of the filter against the vessel wall12,13. Based on our experience with the GARDEX EPD, two of these problems are addressed. First, optimal wall apposition can be achieved by placing the filter into a straight segment of the ICA. This is feasible since the GARDEX EPD can be locked anywhere along the guidewire and anywhere along the vessel, which makes it possible to place the filter freely into a suitable segment. Second, the GARDEX EPD is delivered only after a 0.014’’ wire of choice has been placed in the target vessel. This feature is appealing, especially in challenging lesions with difficult vascular anatomy. Depending on the complexity of the lesion, a pre-placed wire of choice facilitates the crossing of the lesion as well as improving the stability and reduces the first cross debris effect. The GARDEX EPD is then delivered over this wire, just like a stent, smoothly riding over the existing guidewire.
Several authors have outlined the influence of vascular anatomical variables and of the periprocedural stroke rate14-16. Scoring systems for anatomic suitability have been developed7,17 and validated18 to identify patients at high risk for CAS. In this case series very challenging vascular anatomy was present in more than a third of the patients and none of the neurological adverse events occurred in this patient group. So far, there is no evidence favouring any EPD in normal or challenging anatomies. There have been numerous studies establishing the safety of individual devices19-25. Nonetheless, it is impossible to draw any conclusions on the relative effectiveness of particular EPDs because of differences among studies with respect to patient-related factors, operator-related factors and other factors that could affect outcomes. The ideal test to determine EPD effectiveness would be a comparative, randomised trial involving non-protected stenting and protected stenting, which is unlikely ever to be conducted for ethical reasons4.
Two patients had flow-limiting vessel spasm at the site of the filter. In both cases spasm resolved after removal of the filter. Data published so far suggest the occurrence of filter-induced spasm in 7.8%-22% of patients25. In one retrospective study of 414 patients undergoing CAS with embolic protection, the occurrence of filter-induced flow limitation was associated with a higher 30-day stroke and death rate (9.5% vs. 2.9%)26. This highlights the importance of avoiding filter-induced ICA flow impairment. A distinction is made between flow impairment due to spasm and due to filter obstruction as a result of plaque material in the filter. Some authors argue that occlusion of the pores in the filter membrane may be responsible for the impairment in antegrade flow, and that the degree of debris obstruction varies according to the pore size26. Although this seems intuitive, there is not enough evidence yet to support this hypothesis. In any case, filter obstruction was not seen in our case series at all.
The 30-day stroke and death rate was 2.6% in this cohort. There was one periprocedural minor stroke, and there were no deaths and no strokes in the follow-up period. Based on CEA trials, an acceptable upper limit of perioperative stroke or death is 3% in asymptomatic and 6% in symptomatic patients27. In our unselected patient cohort these rates were 0% in symptomatic and 2.9% in asymptomatic patients. This fact underlines the safety of CAS under embolic protection with the GARDEX EPD.
The first experience of CAS under embolic protection with the GARDEX System appears encouraging. Our data suggest that the use of the GARDEX EPD is simple, and shows high success rates even in challenging anatomies. No difficulties were observed in placing and retrieving the filter. The ability to cross the lesion over the guidewire of choice and then deploy the filter in any desired location across the wire creates a unique, natural and appealing advantage, especially in difficult anatomical settings. Clinical outcomes appear to be favourable. The main limitation of this report is the small sample size, which makes it impossible to draw comparisons with other filter devices or other embolic protection systems. Thus, the role of this new device in CAS needs to be further confirmed in a larger patient population.
Conflict of interest statement
D. Scheinert is a consultant for Gardia Medical. U. Rosenschein is a co-founder of Gardia Medical. The other authors have no conflicts of interest to declare.

