
Introduction
Data on perioperative outcomes of new mesh-covered carotid stents have been presented, showing encouraging results1,2. Major complication rates in CGuard carotid artery stenting (CAS) procedures range from 0 to 2.5% at 30 days1,2. These data underline the feasibility and safety of the CGuard™ stent (InspireMD, Tel Aviv, Israel) in preventing off-table events in the so-called plaque healing period, that is to say after the intraoperative embolism risk has been overcome. The IRON-Guard registry2 has continued to collect data from the first 200 patients treated by CGuard stent implantation. The present paper reports the one-year follow-up data completed by all patients.
Methods
Two hundred consecutive symptomatic (8.5%) and asymptomatic patients were prospectively enrolled to be submitted to protected CAS by CGuard stent implantation at 12 experienced vascular centres2 (Supplementary Appendix 1).
At follow-up, neurological carotid-related complication occurrence was evaluated by a treating physician3 as well as external carotid occlusion and in-stent restenosis rates using duplex ultrasound (US) (Figure 1).
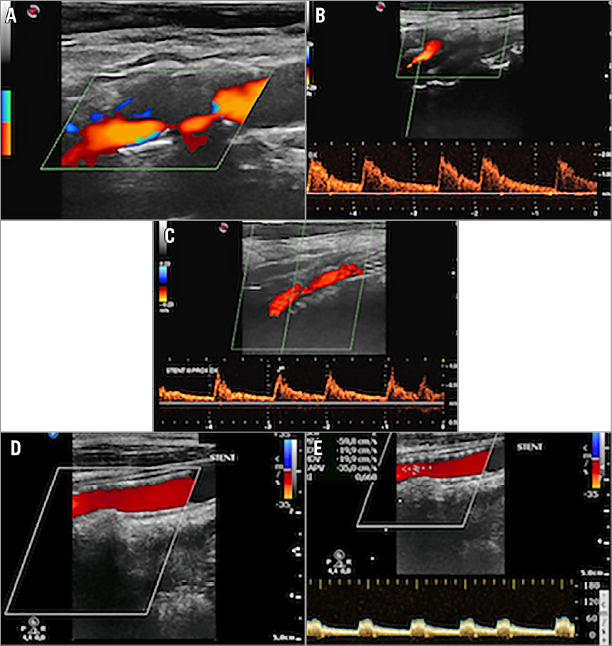
Figure 1. CGuard stent implantation in a severe stenosis. A) & B) Preoperative detection by duplex ultrasound. C) One-month post-procedural assessment. D) & E) One-year control.
All treated patients were submitted to neurologic assessment at one month postoperatively, and to a follow-up clinical visit and US at 1, 3, 6 and 12 months postoperatively.
Results
Dual antiplatelet therapy was maintained for at least 30 days post-procedurally, and lifelong single antiplatelet therapy was prescribed. By 30 days post implantation, one patient suffering a minor periprocedural stroke was submitted to stent explantation because of thrombosis due to ineffective heparinisation2 and was excluded from subsequent per-protocol analysis. All remaining patients (199 out of 200) complied with the 3-, 6-, and 12-month evaluation protocol with no other stent thrombosis observed. No major neurological adverse event, stent thrombosis or external carotid occlusion was recorded from one to 12 months postoperatively; one myocardial infarction was registered at 12-month follow-up.
One asymptomatic restenosis >70% was detected in one patient at three-month follow-up carotid duplex US (peak systolic velocity [PSV] 450 cm/sec). The patient was submitted to a stent-in-stent procedure by new CGuard implantation (9x40 mm) with a residual stenosis <30%, and no neurological sequelae. He was followed up at six and 12 months by US (PSV 220 and 189 cm/sec, respectively) (Supplementary Appendix 2).
Discussion
Data from recent series on protected CAS have shown that periprocedural neurological events can be minimised by the proper and skilled use of embolic protection devices4. Nevertheless, the post-procedural period might still be burdened by non-negligible rates of CAS-related events. Interesting studies have demonstrated the relation between neurological complications and carotid plaque prolapse through the stent struts. Therefore, new mesh-covered carotid stents have been developed and used in many centres with more than encouraging results1,2. A recent meta-analysis by Sannino et al5 reported a 30-day 0.02% event rate for both CGuard and Roadsaver patient groups, thus underlining the safety and feasibility of mesh-covered CAS procedures in different lesion types and vessel anatomies in a cohort of 635 patients.
In our series, we reported no neurological CAS-related complications at 3, 6, and 12 months, in accordance with the 2.5% post-procedural minor stroke rate previously reported2, thus confirming that a mesh-covered stent may stabilise any debris or embolic particles of the carotid plaque, even in highly embologenic ones, from the moment of stent opening until the completion of the plaque healing period and beyond, namely three months after the procedure, when it is supposed that the stent endothelialisation is completed6. Moreover, no stent thrombosis or external carotid occlusion was registered, thus substantiating the hypothesis that mesh-covered stents are safe and effective in treating atherosclerotic lesions encountered at a carotid bifurcation and of internal origin (Supplementary Appendix 3).
The ghost of restenosis seems to have been scared away in new-generation covered stenting: the 0.5% rate of restenosis at one year in the present series is well below the 6.5% rate reported by Szolics et al7 in 2010 and the 38% by Schillinger et al8 in the 2006 (stopped) randomised trial on the use of covered versus bare stents in CAS. Recently, Yilmaz et al9 described a potentially increased rate of occlusion in one particular dual-layer stent design (Roadsaver®, double metal layer design; Terumo Corp., Tokyo, Japan) compared with conventional ones, but analysis of the overall data suggests that this is likely to be design-specific, as the CGuard (PET mesh-covered) design showed no increased propensity for stent thrombosis10. This is probably consistent with the potential relevance of differences in dual-layer stent design11.
In our series, we recorded one single case of restenosis that occurred three months post-procedurally. In that patient, post-dilatation balloon diameter and pressure (respectively, 5 mm, 6 atm for 10 seconds) were in line with common practice, so that we can only speculate that stent underexpansion or malapposition might have been responsible for the very early restenotic lesion. Even if stent strut malapposition occurred in up to 20.5% in a recent CGuard series, it has been reported recently that it can be optimally minimised by appropriate post-dilatation in mesh-covered open-cell stents6. The reported low restenosis rate in our series corroborates the hypothesis that the tissue-friendly structure of the CGuard stent allows a proper growth of intimal layer through the mesh pores to cover the plaque completely with no abnormal hyperplasia. Nevertheless, the effect of balloon pressure on the intima once it has been covered by the mesh and the stent struts should be carefully evaluated. Possibly, once the matter of intraprocedural microembolism from other sources (e.g., access vessels) has been overcome in CAS, future studies will focus on carotid wall pressure sensitivity and reaction in order to minimise long-term restenosis complications due to intimal hyperplasia following CAS.
Limitations
Our study has the limitation of being a single-arm study with no control group; nevertheless, it reflects a real-world experience, given that it collected data from 12 experienced vascular centres performing CAS. Another limitation is the lack of quantitative vascular angiography analysis and of an external core lab.
Conclusions
The CGuard MicroNet™ covered embolic prevention stent has proven to be effective in preventing carotid-related neurological events in both the short-term and midterm results.
| Impact on daily practice The IRON-Guard registry has confirmed the role of the CGuard MicroNet covered embolic prevention stent in lowering CAS-related neurological complications at 12-month follow-up, even in high-risk composition carotid plaques. |
Acknowledgements
We thank all physicians in the participating centres for their contribution to the study (Supplementary Appendix 4).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Appendix 2. Results.
Supplementary Appendix 3. Discussion.
Supplementary Appendix 4. Acknowledgements.
To read the full content of this article, please download the PDF.

