Abstract
Aims: Our aim was to report techniques and outcomes of our experience in percutaneous stenting of ostial common carotid artery lesions.
Methods and results: We retrospectively reviewed patient medical records at our institution from January 2005 until April 2011 to determine baseline characteristics, procedural details and follow-up data of patients who underwent percutaneous stenting of ostial lesions of the common carotid artery. Our study included 17 patients of whom eight (47%) were male. Sixteen of the 17 (94.1%) procedures were performed in left common carotid arteries. In seven recent patients we used a standardised technique, which was characterised by crossing the lesion with a 0.014” wire, followed by insertion of an embolic protection device with a 300 cm long wire. In this technique, the stent mounted on a 0.035” balloon catheter was deployed on both wires instead of only on the embolic protection device wire. Mean follow-up was 17.6 months. During the follow-up, only one patient had a transient ischaemic attack, and none of them had either stroke, ischaemic retinal event, myocardial infarction or contrast nephropathy. Death was observed in five patients at the end of the study period.
Conclusions: Stenting of ostial lesions of the common carotid artery appears safe and effective.
Introduction
A number of studies have compared the safety and efficacy of carotid stenting with carotid endarterectomy (CEA) in significant obstructive lesions of the internal carotid artery (ICA)1-4. With increased experience, better patient selection, improved technology and routine use of embolic protection devices (EPD), outcomes of ICA stenting have improved5. A meta-analysis comparing ICA stenting with and without use of an EPD has shown significantly better outcomes with an EPD6. Although the technique and outcomes of internal carotid artery stenting have been extensively studied, the treatment technique and outcomes of ostial common carotid artery (CCA) disease have not. A surgical option for such a disease includes in many circumstances carotid subclavian bypass8-10. Despite substantially improved results with cervical reconstruction techniques to repair supra-aortic trunk lesions, periprocedural stroke and death rates are as high as 8%11-13. Carotid stenting may provide a safer and less invasive treatment option for patients with ostial lesions of the CCA.
To date, several small studies have reported the outcomes of percutaneous stenting in supra-aortic trunk lesions14-20. Only two of these reports were dedicated to common carotid artery lesions14,15. In both studies, CCA stenting was performed without using embolic protection devices. This stenting technique is less standardised because these lesions are less common, and there are several technical challenges including difficulty in engaging and crossing the lesion without disrupting it and obtaining sufficient mechanical support for delivery and accurate stent placement into the CCA ostium. We report the techniques and outcomes of percutaneous stenting of ostial CCA lesions in consecutive patients undergoing common carotid ostial interventions at our institution.
Methods
Study population
We retrospectively reviewed patient medical records at our institution from January 2005 until April 2011 to determine which patients underwent primary stenting to carotid artery due to carotid artery stenosis (no.=548). We included all patients with percutaneous stenting of ostial lesions of CCA in the study population (17 patients and a total of 17 procedures). Baseline patient characteristics and clinical follow-up variables were obtained from medical records after the Cleveland Clinic Institutional Review Board approved the study protocol. Age, gender, body mass index, previous neurologic events and history of smoking, hypertension, diabetes, hyperlipidaemia, atrial fibrillation, coronary artery disease, coronary artery bypass graft surgery, neck malignancy, radiotherapy, carotid surgery and carotid stenting were noted as baseline characteristics. Preprocedural carotid artery Doppler studies were reviewed to determine the location and severity of the common carotid artery stenosis.
Procedures
We reviewed patient charts, angiography reports and angiographic images for procedural details, location and severity of the stenosis, details about the devices used during the procedures (catheters, wires, angioplasty balloons, stents, EPD), other concomitant procedures and angiographic results of the procedures (TIMI flow). All procedures were performed in the Cleveland Clinic Sones Cardiac Catheterization Laboratory using retrograde femoral artery access. All patients received intravenous heparin for anticoagulation. After the procedure, all patients received clopidogrel for 30 days and aspirin long term.
Follow-up data and outcomes
Patients’ medical records were reviewed for postprocedural complications recorded as death, stroke, transient ischaemic attack (TIA), acute myocardial infarction, retinal embolic event, nephropathy (more than 1 mg/dl rising in creatinine after the procedure), and bleeding. Postprocedural carotid Doppler studies were reviewed to determine the procedural success. In order to assess the restenosis, the velocity in the common carotid artery, distal to the stent, was compared with the velocity in the contralateral common carotid artery. Compared to the contralateral common carotid artery, a more than 20% decrease in velocity was considered as restenosis. We searched the medical records to determine long-term outcomes of restenosis, repeat stenting, other carotid artery stenting, death, stroke, TIA and retinal embolic events. We searched the Social Security Death Index on 21 April 2011, to determine mortality outcomes including all deaths until that date. Death within a month of CCA stenting was defined as 30-day mortality.
Statistics
Data were entered into an Excel spreadsheet and imported into a JMP Statistical Discovery Software (SAS Institute, Cary, NC, USA) program for analysis. Continuous variables were expressed as mean±standard deviation and were analysed using the Student’s t-test. Categorical variables were analysed using the Chi-square test.
Results
Baseline characteristics
Baseline characteristics of patients are presented in Table 1. In our study, eight patients were male (47%), and mean age was 65.1±9.3 years. Four patients had a history of previous stroke, five had previous TIA and one patient had previous amaurosis fugax. Two patients had persistent neurologic symptoms. Only one patient presented to hospital with TIA at the time of procedure. In 16 patients the procedures were elective. Among these elective procedures one patient had recurrent TIA, one patient had recurrent amaurosis fugax, one patient had recurrent syncopal episodes and another patient presented for left heart catheterisation due to recurrent chest pain. Three of 16 patients underwent elective carotid stenting before balloon aortic valvuloplasty; whereas another patient had the elective procedure before concomitant surgical aortic valve replacement and coronary artery bypass graft. Atherosclerotic risk factors (hypertension, hyperlipidaemia, diabetes and history of smoking), peripheral vascular and coronary artery disease were common in our study group. Only one patient had atrial fibrillation. Six patients had previous coronary artery bypass graft surgery. Mean body mass index and mean creatinine were 28.3±5.6 and 1.0±0.3, respectively. Four patients had a history of previous radiotherapy to the neck or upper chest due to different malignancies. One patient had previous contralateral CEA and none had previous ipsilateral CEA.

Procedural details
Procedural details are shown in Table 2. Sixteen (94.1%) procedures were performed in the left CCA. The severity of stenosis was 60-79% in one patient (5.9%) and over 80% in 16 patients (94.1%).
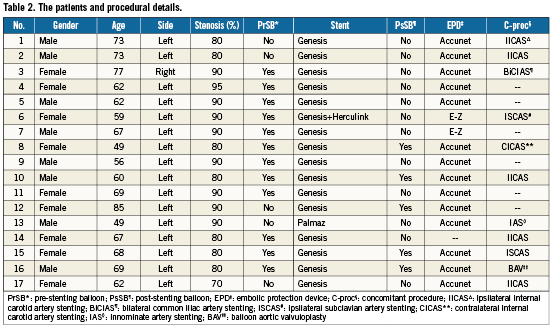
In patient 1, a 70% lesion in the common carotid artery was engaged with a diagnostic catheter telescoped through a guide, and a 0.035” wire was used to advance the guide through the lesion in the common carotid artery to stent the ICA. An EPD was placed along with a 0.014” buddy wire. After performing ipsilateral ICA stenting, the guide catheter was brought back in the aorta over the EPD and buddy wire. Subsequently, a stent was deployed at the CCA ostium only on EPD wire jailing the buddy wire. Once the EPD was captured, the buddy wire was removed after stent deployment. In patients 2, 3, 4 and 6, the lesion was wired with a 0.014” wire from the aorta using a guide. An AL-1 guide was modified by using boiling water to straighten the distal tip in these patients to allow access to the carotid artery without engaging the lesion. The lesion was then crossed with an EPD. The stent was deployed only on the EPD wire jailing the 0.014” buddy wire. After capturing the EPD, the buddy wire was removed. Patient 2 underwent ipsilateral ICA stenting before CCA stenting. In patient 5, the lesion was crossed and stented only on an EPD wire without using a buddy wire, using a guiding catheter. In patient 7, the lesion was first crossed with 0.035” wire, and an EPD was placed after removing the 0.035” wire. The stent was deployed only on an EPD wire without a buddy wire. In patient 8, the lesion was crossed with an EPD wire. ICA stenting was performed first, and subsequently CCA stenting was performed only on an EPD wire without buddy wire, with the guide in the aorta. In patient 14, the lesion was crossed with a 0.035” wire and stented only on this wire without using an EPD. After CCA stenting, the sheath was advanced through the stent to accomplish ICA stenting using an EPD. In patients 10-13 and 15-17 the technique was standardised, so a modified Amplatzer left 1 (AL-1) guide catheter was employed and a 300 cm long 0.014” wire was used to cross the lesion. This was followed by an EPD with a 300 cm long wire. After predilation, a stent mounted on a 0.035” balloon catheter was deployed on both wires instead of only on the EPD wire. This system provided reliable mechanical support for accurate placement of the stent in the ostium and allowed easy retrieval of the EPD after the procedure. The buddy wire was removed last. Patient 17 underwent ipsilateral ICA stenting before CCA stenting using a similar technique.
Predilation was performed in 12 (70.5%) patients. All but one patient required only a single stent. Balloon expandable stents were used in all patients (16 Genesis, one Palmaz [both: Cordis, Johnson & Johnson, Warren, NJ, USA] and one Herculink [Abbott Vascular, Redwood City, CA, USA]). Five patients required post-deployment dilation with a larger balloon. Accunet (Abbott Vascular) was used in 14 patients and FilterWire E-Z™ (Boston Scientific, Natick, MA, USA) in two patients. In one patient (number 14) the procedure was performed without an EPD. All patients but one had less than 20% residual stenosis at the end of procedure; patient 14 had 30% residual stenosis.
Concomitant procedures
Table 2 shows the concomitant procedures during CCA stenting. Ipsilateral internal carotid artery stenting (five patients), contralateral internal carotid artery stenting (one patient), ipsilateral subclavian artery stenting (two patients), innominate artery stenting (one patient), bilateral common iliac artery stenting (one patient) and balloon aortic valvuloplasty (one patient) were among the concomitant procedures.
Doppler ultrasound data
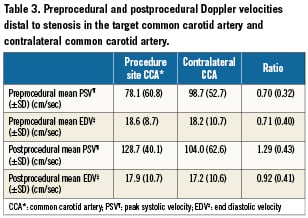
Table 3 shows the pre- and postprocedural Doppler velocities distal to the stenosis in the target and contralateral CCA. In the target CCA, peak systolic velocity (PSV) was lower compared to PSV in the contralateral CCA (78±61 cm/sec versus 99±53 cm/sec, p=0.10), but the difference did not reach statistical significance. Mean PSV in the target CCA significantly increased after the procedure (129±40 cm/sec, p=0.013).
Procedural and 30-day outcomes
One patient (number 4) had a TIA with localisation to the contralateral side on the fourth postprocedure day. The patient had right facial drop and difficulty with speech that resolved in four to five minutes. The patient presented to the emergency room in another hospital. The head computerised tomography was normal. Afterwards the patient stayed event free during the follow-up. None of the patients had stroke, amaurosis fugax, myocardial infarction, or contrast nephropathy within 30 days postprocedural. Patient 16 had femoral haematoma after the procedure. All patients survived more than 30 days after the procedure.
Long-term outcomes
Mean follow-up was 17.6 months (range 1 to 50.5). During the long-term follow-up, none of the patients developed restenosis, stroke, TIA or ischaemic retinal event, but five patients died (numbers 1, 7, 9, 11 and 12). All of the patients were free of neurologic symptoms during first six months. During the follow-up period, three patients (numbers 11, 12 and 16) required balloon aortic valvuloplasty, one patient (number 15) underwent coronary artery bypass graft surgery and one patient (number 13) underwent aortic valve replacement and coronary artery bypass graft surgery. One death was due to sudden cardiac death (after 36 months), and four deaths were due to unknown reasons (after 23, 34, 47, and 59 months).
Discussion
In this study, we present our experience in stenting of CCA in 17 consecutive patients undergoing ostial common carotid interventions. All procedures were successful with one TIA and no stroke, retinal ischaemic event, myocardial infarction, or contrast nephropathy at 30 days. We did not observe any remote neurological event at follow-up. There were no deaths within one year of intervention. The five deaths during long-term follow-up occurred between 23 and 59 months after the procedure. None of our patients required repeat interventions due to restenosis.
The safety and efficacy of carotid stenting in significant obstructive lesions of the internal carotid artery (ICA) have been investigated in several large studies1-4,7. The CREST study is the most recent randomised controlled study to show similar short- and long-term outcomes with stenting compared to CEA outcomes7. Improved technologies, routine use of an embolic protection device, better selection of patients and, finally, increased experience have led to these encouraging results5.
Repair with cervical reconstruction techniques (subclavian-carotid, carotid-carotid, or carotid axillary) is still the main approach for obstructive lesions of the CCA21,22. A review of 100 consecutive supra-aortic trunk reconstructions during 16 years in one centre showed periprocedural rates of stroke (8%), death (8%) and myocardial infarction (3%)14. Furthermore, cervical reconstruction techniques sometimes require thoracotomy or median sternotomy. These limitations compel clinicians to seek a less invasive approach in treatment of supra-aortic trunk lesions.
Several studies have reported outcomes of percutaneous stenting of supra-aortic trunk lesions14-20. Queral et al reported results of 26 stenting of aortic branch lesion procedures in 22 patients with six of the procedures in the CCA19. The researchers used surgical exposure of the CCA and clamping of the distal CCA before percutaneous retrograde deployment of a CCA stent to prevent any distal embolisation. During the 27-month follow-up period, there were no strokes or deaths. Sullivan and co-workers reported their experience in angioplasty and stenting of supra-aortic trunk lesions20. Among a total of 83 patients, 14 procedures were performed in the CCA. Three patients had iatrogenic dissections during the interventions on the CCA. Furthermore, two patients had stroke after the procedure. Another study reported results of 42 procedures of ostial and proximal CCA stenting in 37 patients14. In this study two strokes and one death (due to retroperitoneal bleeding) were observed during the postprocedural period. Long-term outcomes revealed one TIA, two minor and one major stroke. Procedural success was 95% and during the 24-month follow-up period, the restenosis rate was 5.1 %. In a very recent study, authors reported outcomes of angioplasty and stenting of the CCA in a total of eight procedures and seven patients15. Seven of eight procedures were successful. Procedural TIA was observed in two patients. During the 31.7-month follow-up, two TIA and no strokes were observed. An EPD was not used in any of these studies. Another small case series reported results of nine CCA and a total of 20 supra-aortic trunk stentings18. During this series, the authors used surgical clamping of the distal CCA or EPD for cerebral protection. There was no stroke, TIA, myocardial infarction or death during the 30-day follow-up.
Our study is the first report where an EPD was used routinely (except one procedure) for CCA stenting. Our short- and long-term results were better compared to previous studies despite our study group consisting of high-risk patients as evidenced by their concomitant procedures and comorbidities, and four patients with previous radiotherapy14-20.
One of the major concerns during CCA stenting is distal embolisation during the procedure. Clinicians have made great efforts to try to minimise the risk of embolisation. In early studies, surgical exploration of the CCA and external clamping of distal CCA before percutaneous retrograde stenting of the CCA were tried to lower the risk of embolisation9,23. With routine use of an EPD, the risk of embolisation has been significantly lowered in carotid stenting procedures6. However, the risk of distal embolisation is still a major concern, especially while passing ostial proximal lesions of the CCA. In many patients we used a “no touch” technique where the lesion was not crossed with a guiding catheter. Modification of the guiding AL-1 catheter where the tip is straightened with heat application allowed us to place the catheter pointing towards the carotid ostium. Initial use of a 0.014” wire to cross the lesion allowed for easy and atraumatic passage of an EPD through the lesion. Use of both wires to advance and place a stent is another technique innovation where adequate support and precision are reliably achieved. Moreover, the shape of the guide, buddy wire and precise stent placement allow easy and reproducible retrieval of the EPD.
Patient CCA velocity can vary considerably24-26. Causes of variability in CCA velocities may be related to several factors such as vascular geometry, vessel wall compliance, and haemodynamic parameters like heart rate, blood pressure and cardiac output26. Therefore, simply using a cutoff value to substantiate CCA stenosis would be misleading. To overcome this problem during the diagnosis of CCA stenosis, we compared the PSV and end-diastolic velocity (EDV) in the target CCA with PSV and EDV in the contralateral CCA. Our results indicate many patients with common carotid lesions have decreased peak systolic velocity distal to stenosis compared to the contralateral side. This velocity tends to increase and match the contralateral side after successful stenting.
Our study is a single centre study and does not compare endovascular with surgical treatment. Another main limitation is the small patient number. Procedural technique varies depending on operator and lesion characteristics. Our mortality data were obtained from the Social Security Death Index, and we were unable to determine the reason of death in four patients.
In conclusion, with technology improvements and routine use of EPD, the outcomes of CCA stenting are promising in an experienced centre. CCA stenting, even in high-risk patients, appears safe and effective. Use of a “no touch” technique to negotiate the EPD through the lesion, use of a buddy wire and stent deployment over both the EPD and a buddy wire provided a reproducibly safe and effective approach to common carotid artery stenting.
Conflict of interest statement
The authors have no conflict of interest to declare.

