Abstract
Aims: The aim of the present study was to evaluate the outcome of carotid artery stenting (CAS) in a single, high-volume centre of a single operator and to analyse the circumstances under which complications occur. Recent trials comparing CAS with carotid endarterectomy demonstrated controversial results. The low experience of interventionists in performing CAS was a major limitation of these studies. The number of procedures needed to achieve optimal skills is unknown.
Methods and results: From May 1997 until April 2010, 1,004 patients with symptomatic or asymptomatic carotid artery stenosis underwent CAS by a single operator. A cerebral protection device was in mandatory use since 2000. In-hospital complication rates were defined as the cumulative rate of death, myocardial infarction or stroke. Procedural success was achieved in 97.77% of patients. The perioperative complication rate was 1.69% including 0.2% deaths, 1.1% patients with minor stroke, 0.4% patients with major stroke. In 88% (15 out of 17) of the patients with complications, unfavourable anatomical or procedural factors could be identified. After the first 100 CAS performed,the complication rate was at 3% and significantly decreased to 1% after more than 500 procedures. Patients ≥ 80 years had a significantly higher complication rate.
Conclusions: In a high-volume experienced centre, the in-hospital complication rate is low. Complications occurred almost exclusively in patients with unfavourable anatomical or procedural characteristics and seem to be avoidable in most patients. A learning curve was observed up to 500 procedures. Elderly patients have a higher complication rate.
Introduction
Stroke is the third leading cause of death in industrialised countries and the major cause of functional impairment1. Most strokes are ischaemic, caused by atherosclerotic emboli from the carotid artery.
The incidence of asymptomatic relevant stenosis of the carotid artery increases in older age and is found in 5-10% of persons >65 years2. The annual risk of stroke is between 1-4% in patients with asymptomatic stenosis and correlates with the degree of stenosis3,4. In symptomatic patients, the risk of stroke is higher with 30% within three month after a transient ischaemic attack (TIA)5.
Carotid endarterectomy (CEA) has been established as the preferred treatment to prevent stroke for symptomatic and asymptomatic patients with significant stenosis of ≥50% compared to medical therapy6-8. The treatment is only effective if the perioperative risk of death and stroke does not exceed 3% in asymptomatic and 6% in symptomatic patients9,10.
In the last 10 years carotid artery stenting (CAS) has emerged as a less invasive alternative to surgery. Four large randomised controlled trials have compared the effectiveness and safety of CAS with CEA. Three of these trials have enrolled only symptomatic patients11-13, whereas the most recently published study included symptomatic as well as asymptomatic patients14. In the EVA-3S and in the ICSS trials, CAS was significantly inferior to CEA12,13, in the SPACE study11, the non-inferiority endpoint was not met. In all three studies there were obvious reasons for the worse outcome in the CAS group. For instance, cerebral protection was only used optionally. Most importantly, however, the majority of the interventionists were inexperienced. Only five to 10 procedures were required in order to participate in the studies. In contrast to these trials, in the CREST study14, cerebral protection was mandatory and the interventionists had to undergo a training phase before they could enrol patients in the randomised trial. As a consequence, CREST is the first large randomised trial which showed no difference in the 4-year outcome between both treatment modalities (periprocedural stroke, myocardial infarction, death or any ipsilateral stroke within four years after randomisation 7.2% for CAS and 6.8% for CEA).
In contrast to CEA, CAS is a relatively new interventional procedure which is still under development and is unavoidably performed by a significant number of inexperienced interventionists. The learning curve for CAS has been evaluated in single centres and for single operators. To reach a threshold of 3-5% for periprocedural major adverse cardiac and cerebrovascular events (MACCE), 70-100 procedures were necessary15,16. Whether the MACCE rate could be further decreased by increasing the number of procedures per operator has never been analysed.
The purpose of the present study, therefore, was:
– to analyse the procedural outcome in a large cohort of patients in a single, high-volume centre using state of the art techniques by a single operator, and to describe the reasons for treatment failures
– to evaluate the number of procedures needed by a single operator to achieve the optimal skills (individual learning curve)
– to identify subgroups of patients at high risk for procedural complications.
Methods
Patients
Between May 1997 and April 2010, 1,004 consecutive patients with carotid artery stenosis underwent carotid artery stenting (CAS).
The indications were:
– Stenosis ≥60% of the luminal diameter by angiography for symptomatic patients.
– Stenosis ≥80% of the luminal diameter by angiography for asymptomatic patients.
Contraindications were:
– Complete occlusion of the carotid artery.
– Floating thrombus in the lesion.
– Vascular disease precluding use of a catheter-based technique.
– ≥ 80% intracerebral stenosis of the target vessel.
– Ischaemic stroke within 48 hours.
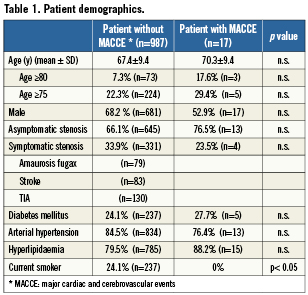
The indication was confirmed by an independent neurologist, who examined the patients before and within 24 hours after the procedure. Written informed consent was obtained from each patient. The demographic data of the patients are summarised in Table 1.
Procedure
The intervention was performed under local anaesthesia via the femoral artery. A long sheath was positioned in the common carotid artery below the bifurcation and 5000 IU of heparin and 0.5 mg of atropine were given. A cerebral protection system was in mandatory usage since its availability in 2000, and was either an occlusive protection device which blocks the blood flow in the target vessel during the procedure, or a filter.
In case of a ≥95% stenosis (according to the NASCET criteria), the lesion was predilated followed by the implantation of a self-expending stent and a postdilatation according to the diameter of the vessel. Upon conclusion of the procedure intracerebral angiograms were obtained in two perpendicular projections. Patients were monitored for at least 24 hours and underwent neurological examination before discharge. Dual antiplatelet therapy was given for four weeks.
Definitions
Patients were considered as “symptomatic” if they had an ipsilateral neurological ischaemic event within the last six months.
“Procedural success of carotid artery stenting” was defined as residual stenosis ≤ 30% and the absence of complications.
“The in-hospital complication rate” was defined as the in-hospital cumulative rate of death, myocardial infarction or stroke.
“Stroke” was defined as a new neurological deficit lasting longer than 24 hours. “Minor stroke” was defined as a neurological deficit which dissolves completely within 30 days, or did not lead to impairment in daily activities as judged by the independent neurologist. “Major stroke” was defined as a persistent neurological deficit leading to impairment in daily activities as judged by the independent neurologist.
“Myocardial infarction” was defined as ≥3 fold CK-increase combined with ECG-abnormalities or typical symptoms.
“String sign lesions” were defined as pre-occlusive with a minimal length of 20 mm.
“Lesion eccentricity” was defined as the eccentricity index >0.7. The maximal (A) and minimal wall thickness (B) were measured on cross-sectional images. The eccentricity index was calculated using the following formula: (A – B)/A.
“Calcification of the target lesion” was defined as positive if angiographically visible.
“Restenosis” was defined as ≥80% stenosis and ≥50% in the treated segment (the stent plus 5 mm on either side of the stent) for asymptomatic and symptomatic patients, respectively.
“Major adverse cardiac and cerebrovascular events (MACCE)” was defined as myocardial infarction, major or minor stroke, or death.
Statistics
Continues variables are expressed as mean ± SD, categorical variables as count and percentages. Group comparisons were made using chi-square test, the ANOVA-test, and the Bonferroni´s Multiple Comparison test, respectively. A p<0.05 was considered as statistically significant. Multivariate logistic regression analysis was performed to identify independent predictors for MACCE. All statistical analyses were performed using the PRISM 3.0 and SPSS version 15.0. statistical software.
Results
Patient demographic and clinical data
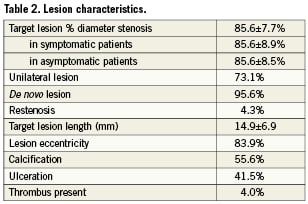
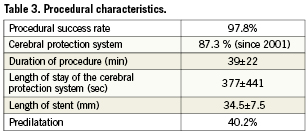
The demographic characteristics of the patients with and without in-hospital complications are summarised in Table 1. The mean age was 67.4 years, 68.2% were male, 7.3% were ≥80 years. One-third of the patients had symptomatic carotid artery stenosis, 24.1% were diabetics. The lesion characteristics are shown in Table 2, the procedural data are presented in Table 3. The procedural success rate was 97.8%. Those patients in whom the procedure failed were sent to surgery.
Periprocedural complications
The overall in-hospital complication rate was 1.69% (n=17), including 11 (1.1%) patients with minor, four (0.4%) patients with major stroke, and two (0.2%) deaths (Figure 1). The complication rate was not significantly different for symptomatic (1.1%) as compared to asymptomatic patients (2.0%; p=0.18, 95% CI 0.99-1.02) (Table 4). In six patients, complications occurred intraprocedural; in 12 patients, complications presented postprocedural before discharge (Table 4). Out of the 17 patients who experienced a complication in 16 the reason could be analysed (Table 5).
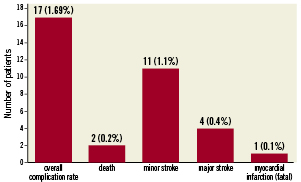
Figure 1. Number and percent of patients with in-hospital complications.
One patient died due to a major stroke followed by a myocardial infarction. This patient was planned for coronary bypass surgery. The second patient who died experienced a hyper-perfusion syndrome, with huge intracerebral bleeding within 24 hours after stenting of a 99% carotid artery stenosis.
Circumstances associated with strokes in the remaining 14 patients were (Table 5):
– Subacute stent-thrombosis due to proven complete clopidogrel resistance in one patient.
– Slow flow through the filter due to debris, which was not aspirated before retrieval in two patients (Figure 2).
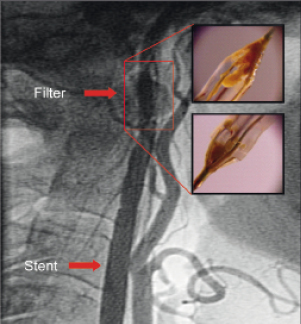
Figure 2. Slow flow phenomenon due to debris after implantation of a stent in the internal carotid artery. Inserted pictures demonstrate the debris captured by the filter.
– String sign stenosis and/or severe tortuosity of >180° in five patients (Figure 3, typical example of a string sign stenosis).
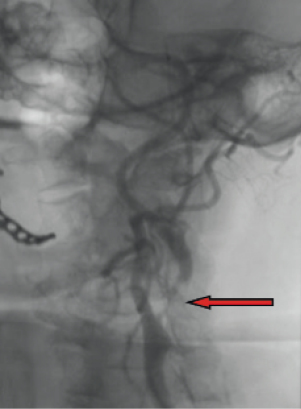
Figure 3. String sign lesion (arrow) of the internal carotid artery.
– Massive ulcerated plaque in a 95% stenosis in one patient (Figure 4).
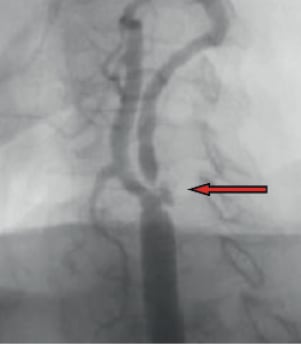
Figure 4. Massive ulcerated plaque (arrow) in a 95 % stenosis of the internal carotid artery.
– High grade long tandem stenosis in one patient.
– Type III aortic arch in two patients with no access to the target vessel (right internal carotid artery) in one patient.
– In two patients, there were no obviously unfavourable anatomical or procedural reasons that could be identified as responsible for the complications.
Multivariate logistic regression analysis
To identify independent predictors for MACCE, a multivariate logistic regression analysis was performed including the following patient and lesion characteristics: age, gender, symptom status, hypertension, diabetes mellitus, dyslipidaemia, lesion length, -severity, -ulceration, -eccentricity, -calcification, and -thrombi. None of these factors were significantly associated with MACCE.
Learning curve
The in-hospital complication rate in the first 100 patients was 3%. This complication rate was reduced significantly to 1% after 500 patients (p<0.05, 95% CI 0.05-3.94), Figure 5.
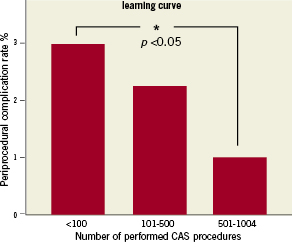
Figure 5. Learning curve.
Subgroup analysis
In the subgroup of patients ≥80 years compared to younger patients, the complication rate was significantly higher (4.11%vs. 1.5%, p=0.04, 95%, CI 0.10-1.26).
Discussion
The main findings of the present study are:
– a procedural success rate of 98%
– a low complication rate of 1.7% compared to randomised trials
– unfavourable anatomical or procedural factors were seen in 88% of the patients who had in-hospital complications
– the learning curve for a single interventionist flattening out after 500 procedures
– a significantly higher complication rate in patients ≥80 years.
The procedural success rate in this single centre series of patients is in the same order of magnitude as described in randomised trials11-14. The in-hospital complication rate of the present study is 1.7%. Compared to the 30-day complication rate found in randomised trials (between 5.2% and 9.6%), this is considerably lower. It is evident from the literature that the difference between the in-hospital complication rate, as assessed in the present study, and the 30-day complication rate, studied in randomised trials, is very small, 80 to 90% of the 30-day complications occurred before discharge17,18.
One reason for the lower complication rate in the present study may be the routine use of cerebral protection devices in our patients, when technically feasible. Although the role of cerebral protection has not been evaluated in controlled randomised trials, meta-analyses have shown a risk reduction by 40%19. Another reason for the low complication rate in the present registry may be the high operator experience, which could be gained over a time period of more than 10 years. Centres with similar experience have comparable complications rates20,21.
In randomised trials the complication rates among interventionists of different training levels were not different11-13. In these trials, the definition of skill was arbitrarily set at 25 procedures, which is not based on scientific data. Other investigators have shown that skill set for CAS continues to increase beyond 25 procedures. Verzini et al23, for instance, found a stable risk-rate for major stroke of less than 2% only after 195 procedures. Similarly, Lin et al24 reported a significant reduction in the stroke and death rate after 150 procedures; a similar learning curve was also seen in another large registry21. In the CASES-PMS study22 a comprehensive learning program was needed to gain an acceptable safety and efficacy outcome, which was further supported by a meta-analyses of different case series. In active carotid artery stenting units it took almost two years before the stroke/death rates fell below 5%23. The present learning curve for periprocedural complications (death, major and minor stroke, and myocardial infarction) flattened only after 500 procedures, resulting in an overall complication rate of 1%.
We decided to analyse under which circumstances the procedure failures occurred and how they influenced the learning process. A detailed analysis of the patients who experienced an in-hospital complication revealed unfavourable anatomical or procedural characteristics in almost 90% of these patients. In retrospect, most of the complications seemed to be avoidable, there was no obvious anatomical or procedural risk factors present in only two out of the 14 patients who suffered a stroke.
In two other patients with stroke, a slow flow through the filter was observed, and no aspiration was performed. This happened at the very beginning of our filter experience (January and February 2000). After this finding, we started to aspirate the debris in this particular situation, as reported in the literature24 and never saw a similar stroke-event.
In five patients, a string sign stenosis and severe tortuosity, a well-known high risk lesion for CAS, had been treated with a filter protection system. After this bad experience, we never touched an asymptomatic lesion like that again, and started using a proximal protection system for symptomatic patients which has been shown to be associated with low complication rates27. The same is true for patients with massive ulcerated plaques and long tandem stenosis, where a large amount of debris may exceed the capacity of a filter.
Another constellation prone to higher risk is a type III aortic arch, which was present in two of our patients with complications. Although we prefer long flexible sheaths in most cases, in patients with type III arches we have used guiding catheters since we realised that the access to the carotid artery is much easier and faster, and thus never experienced an adverse event again.
In patients ≥80 years as compared to younger patients we observed a significantly higher complication rate, which is also described in the literature18,25. Based on other, as well as our own experience, elderly patients are more likely to have tortuous vessels, more complex aortic arches, and complex carotid lesions26.Those patients seem to do better with CEA14, although it remains to be proven whether the complication rate after surgery falls below 4%, as achieved in our registry.
Clinical implications
In-hospital complications after CAS are mainly seen in patients with unfavourable anatomical or procedural characteristics. For those colleagues who want to start a CAS program, a possible recommendation might be to not treat these specific complex patients at first due to the long duration of the learning curve as observed in our study. In addition, it is important to take into account the different strategies that we described above which may be helpful to prevent these complications.
Limitations
This is a single centre and single operator retrospective evaluation, which is valuable but scarcely applicable in most centres. Moreover, data on in-hospital complications, and not on 30-day complications, are given.
Conclusions
The present study results suggest that CAS is an effective and safe alternative to CEA when performed in high-volume, experienced centres with complications occurring almost exclusively in patients with unfavourable anatomical or procedural factors. These complications seem to be avoidable in the majority of patients, however, elderly patients were seen to have more in-hospital complications. A flattening of the learning curve was observed only after 500 procedures, when a favourable complication rate of 1% was observed.
Conflict of interest statement
The authors have no conflicts of interest to declare.

