Introduction
This article in the EuroIntervention Tools & Techniques series provides the “how-to-do” plus “tips & tricks” for carotid artery stenting (CAS). The complete, unabridged e-version with dynamic images can be viewed at www.eurointervention.org. The following is a summary and highlights the key approach that underpins a safe practice and the way to obtain good results.
Endovascular carotid intervention was developed due to a need to provide a less-invasive and less-traumatic revascularisation strategy for patients considered high-risk for open surgery. The currents of rapid advancement in endovascular technologies and techniques over the last 10 years has resulted in the evolution of CAS to a refined procedure with great potential to be applied to routine carotid revascularisation practice. The benchmark for perioperative stroke or death for carotid revascularisation is the limit of 6% for symptomatic and 3% for asymptomatic patients.
How to achieve favourable outcomes… Evolutionist concept
To attain good results the multi-factorial CAS strategy is grounded on the following:
1. A “tailored-approach” in the application of endovascular technologies and techniques to a specific-patient with a specific-lesion and vascular anatomy.
2. The choice of stent, embolic protection device (EPD), guiding-catheter and sheath is strongly dependant on an in-depth knowledge of neuro-assessment, carotid plaque characteristics, vascular anatomy and technical features of a vast array of endovascular materials. Following the patient assessment, this information should be integrated to predict the embolic-risk of revascularisation.
3. Experience with a wide range of devices allows the operator the flexibility to choose the most appropriate tools and techniques for the safe application of CAS.
This approach is governed by the results of our study, published in the November 2009 issue of EuroIntervention, assessing the success, safety and long-term durability of CAS in stroke prevention for “all-comers” managed with mandatory neuroprotection and a tailored-approach to intervention.
a. The Cotignola-registry is a prospective registry and the study analysed 1,523 CAS procedures in a population with a very high burden of polyvasculopathy.
b. The defining strength of the data reflects treatment for all-comers with minimal exclusion criteria and a near universal “proceed to endovascular intervention” following conventional diagnostic angiography confirming duplex ultrasound findings; meaning, that more than 99% of patients who required revascularisation proceeded to the endovascular approach.
c. CAS success was 99.6% and regarding early outcome, the 30-day all-stroke/death rate was 1.5%.
This study suggests that very good results can be achieved with i) mandatory neuroprotection; ii) a tailored-approach; and iii) well-trained operators. Emerging technologies and further innovations applied in this way may move us that much closer to the aim of stroke prevention.
Carotid plaque and vascular anatomy evaluations
The evaluation of carotid plaque profile should describe:
1. Degree of stenosis and vessel dimensions.
2. Length/bulk of disease and the morphologic features that predict lesion complexity such as degree of calcification and embolisation-potential (“vulnerable plaque”).
a. Long, irregular or ulcerated lesions (Figure 1) and clinically unstable plaques (recurrent transient ischaemic attacks, TIAs) define a high-risk disease subset.
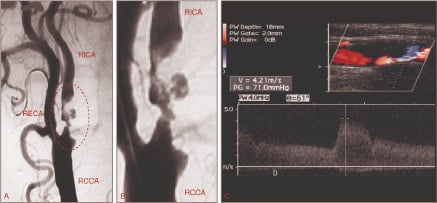
Figure 1. Evaluating the embolic risk of carotid plaques by means of angiography and ultrasound. (A) Angiographic aspect of an ulcerated carotid plaque (circle); (B) Ulcerated portion in detail; (C) Ultrasonographic appearance of a “soft” plaque (large lipid pool covered by thin fibrous cap). RICA: right internal carotid artery; RCCA: right common carotid artery; RECA: right external carotid artery.
b. Plaques characterised by a large lipid pool covered by a thin fibrous cap are more prone to perioperative embolisation as compared to fibrous plaques2. These vulnerable plaques are less echogenic (“soft-lesions”) on B-mode ultrasound and can be quantified by the Grey Scale Median (GSM) method (Figure 1). In the ICAROS study3, the risk of CAS-related stroke was significantly higher in lesions with GSM <25.
The assessment of vascular profile includes defining:
1. Configuration of the aortic arch (Figure 2).
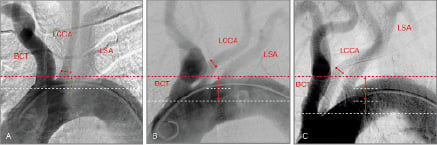
Figure 2. Aortic arch anatomy is classified based on the distance of the origin of the brachiocephalic trunk from the top of the arch. The widest diameter of the LCCA is used as a reference unit (double head arrows). In Type I (A), Type II (B) and Type III (C) arches the brachiocephalic trunk originates within one, within two and more than two diameter lengths (white dotted line) from the top of the arch respectively (red dotted line). This classification points to increasing levels of technical difficulty for catheter engagement, stability and provision of support for intervention as the catheter tends to prolapse into the ascending aorta. LCCA: left common carotid artery; BCT: brachiocephalic trunk; LSA: left subclavian artery.
2. Arch embologenic-risk in terms of burden of irregular, ulcerated and calcified atheroma (Figure 3).
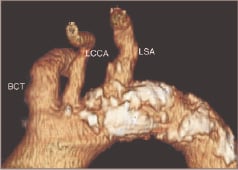
Figure 3. Example of an aortic arch with high embologenic risk. Note the extense, irregular and calcified plaque in the aortic wall (white). LCCA: left common carotid artery; BCT: brachiocephalic trunk; LSA: left subclavian artery.
3. Angulations and tortuosity, coiling and kinking of supra-aortic trunks (Figure 4).
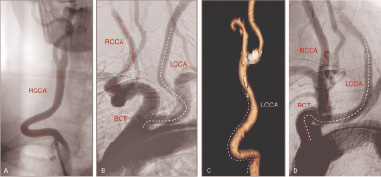
Figure 4. Challenging anatomies of the supra-aortic trunks. (A) Accentuated tortuosity of the RCCA; (B) Tortuosity of both common carotid arteries; (C) Proximal kinking followed by distal tortuosity of the LCCA, (D) Kinking of the brachiocephalic trunk followed by angulated common carotid arteries in a bovine aortic arch. RCCA: right common carotid artery; LCCA: left common carotid artery; BCT: brachiocephalic trunk.
4. Level of carotid bifurcation and its anatomy regarding angle of take-off of the internal carotid artery (ICA), tortuosity at lesion-site and vessel dimensions (Figure 5).
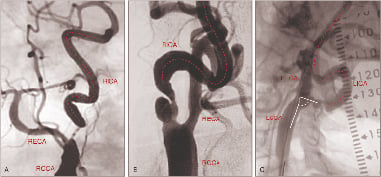
Figure 5. A severely angulated take-off and tortuous proximal segment of the ICA indicate the use of proximal occlusion devices and a highly adaptable stent. (A - B) Accentuated tortuosity of the RICA (indicated by red line); (C) Angulated take-off of the LICA (indicated by white angle) followed by distal vessel tortuosity (red line). RCCA: right common carotid artery; RICA: right internal carotid artery; RECA: right external carotid artery; LCCA: left common carotid artery; LICA: left internal carotid artery; LECA: left external carotid artery.
5. Intracranial segment of the ICA and ipsilateral/contralateral cerebral circulation (Figure 6) to determine collateral flow including circle of Willis and identifying abnormal flow patterns.
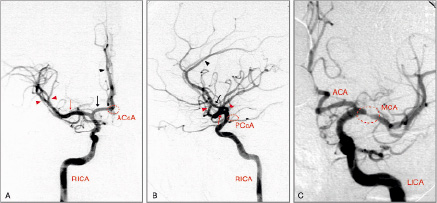
Figure 6. (A) Right AP intracranial angiogram and (B) Right lateral intracranial angiogram. The black arrow points to the anterior cerebral artery leading to the pericallosal artery (black arrow-head). The middle cerebral artery is shown by the red arrow and its branches (red arrow-head). Note the potential source of collateral flow from the contralateral hemisphere through the ACoA (red dotted circle in A) or between the anterior and posterior cerebral circulations through the PCoA (red dotted circle in B). (C) A severe lesion (red dotted circle) at the ostium of the MCA requires treatment before approaching the carotid bifurcation. ACoA: anterior communicating artery; PCoA: Posterior communicating artery; RICA: right internal carotid artery; LICA: left internal carotid artery; ACA: anterior cerebral artery; MCA: middle cerebral artery.
Indications
The indications for carotid intervention include:
1. A symptomatic patient with an angiographic stenosis of ≥50%; that is, a lesion-related neurological event in the preceding six months.
2. An asymptomatic patient with an angiographic stenosis of ≥80%.
The potential factors for considering a patient high-risk for carotid endarterectomy (CEA), thus indicating an endovascular approach, are shown in Table 1. Some of the issues that may prove a challenge or increase the risk of the stenting procedure are outlined in Table 2.
Methods
Extensive discussion and illustration of the tailored-approach, tools & techniques are presented in the web-based version. This issue serves to inform our practice by understanding the tailored-choice of neuroprotection and stent systems.
Accepting that there is no randomised controlled trial (RCT) assessing the efficacy of neuroprotection and that such a study is unrealistic; earlier studies have suggested that the routine use of EPDs results in favourable outcomes4-8. In the Cotignola-practice, neuroprotection is mandatory and such a protocol standardises the procedure which contributes to its effectiveness. Experience is thus maintained at a high-level, an essential element in the safe application of these devices. The choice of distal filter or proximal occlusion systems is highly dependant on many factors such as symptom status, vascular anatomy and plaque characteristics. This practice allows experience to be gained in the use of multiple tools which are readily available on the shelf, allowing the safe application of the individual treatment approach. The results from our recent study firmly add to the evidence that neuroprotection should be the standard of care1.
Understanding the structural and functional characteristics of self-expanding carotid stents allows the operator to tailor the choice of the stent system. The great value of stent design is well demonstrated in the recently reported Cristallo Registry9.
1. An important concept to consider is the view that the type of stent implanted may play a vital part in preventing neurological events due to plaque prolapse. It is appreciated, that, whereas in the open surgical techniques the atheroma and thrombus burden are excised; the stent-protected angioplasty technique compacts this material to the wall, retaining it with its supporting scaffolding and wall-coverage properties.
2. The stent-cell geometry may thus have an “intrinsic anti-embolic property” influencing the risk of plaque-prolapse and distal embolisation during the 24-hour postprocedural and recuperative periods until re-endothelialisation is completed.
3. An extension of this concept, though debatable, is the potential impact of free-cell area on embolisation risk. The free-cell area between the struts of a stent determines vessel scaffolding and wall-coverage properties (Figure 7). As a consequence, a small free-cell area may provide more effective plaque-covering and reduce the risk of disrupted plaque protruding through the interstices of the stent with the potential of embolisation.
Recognising the individual technical characteristics of various carotid stents, it is clear that the interaction between a particular stent and the diseased vessel is unique and no single stent is applicable for all situations. Different stent designs demonstrate functional equivalence when used in uncomplicated scenarios, such as simple supra-aortic anatomies, straight carotid bifurcations and stable fibrous plaques. But, in order to treat all-comers with a breadth of complexity a move toward the tailored-approach is required; and this strategy is supported by the results of our recent study1.

Figure 7. (A) Open-cell design. The free cell area is shown in green; (B) Closed-cell design. The free cell area is shown in green; (C) Straight and tapered-stent configurations.
The section devoted to the step-by-step CAS technique is described in detail including tips & tricks to successfully manage challenging anatomical obstacles. We have depicted the technical aspects representing the day-to-day Cotignola-practice detail built on nearly two decades in the endovascular field. Such practical clinical utility includes, for example, our experience in the application of cutting balloon technology for heavily calcified plaques10. An in-depth account of the prevention and treatment of complications, though uncommon, is reviewed5,11,12. Finally, recorded cases were selected to serve as a hub to this learning tool, designed to help the reader integrate the knowledge of the tailored-approach to CAS.
Consideration
We strongly believe that endovascular carotid intervention may be considered to be a safe therapy, highly feasible and appears to reduce complications to a new threshold. An important element of the tailored-strategy is the recognition of high-risk cases for carotid stenting dependent primarily on the skill of the interventional vascular specialist. This is considerably more relevant in this field than other areas of percutaneous interventions. Thus, it is imperative that the early operator carefully select patients and continue to collaborate with a teacher.

