Abstract
Aims: The aim of this study was to propose new anatomical-procedural classification systems for evaluating carotid lesions and carotid artery stenting (CAS).
Methods and results: The methodology used to propose new concepts to be applied in the carotid district was based on three steps: 1) research in PubMed with the terms “carotid artery” (CA) plus “classification” was performed in August 2010 to verify the existence of any classification system for the CA that could be applied for evaluating CAS; 2) formulation of the “stenting segment” concept and elaboration of two classification systems - (a) the “ABC” system for classifying carotid lesions according to their location, and (b) the “five arterial zones” system for identifying specific arterial zones of the CA concerning the basics steps of CAS; and 3) retrospective testing of the applicability of these classification systems on two hundred and fifty consecutive CA angiograms, in which an obstructive lesion was identified. It was possible to delimit the “stenting segment”, to classify the carotid lesions according to the “ABC” classification system and to identify the arterial zones according to the “five arterial zones” classification system in all (100%) CA angiograms studied.
Conclusions: The “ABC” and the “five arterial zones” anatomical-procedural classification systems are applicable in patients with obstructive disease of the extracranial CA. These systems may contribute to the standardisation of CAS technical evaluation.
Introduction
Carotid artery stenting (CAS) has been used worldwide as an alternative therapy for treating severe carotid stenosis in patients at high risk for carotid artery endarterectomy (CEA)1-5. Definitive parameters for including a patient in a high-risk CEA group1-4,6-9 have been stated but the same is not true for CAS2,4,10-13. CAS reports generally associate the technical difficulties and risks of the procedure with patient age4,7,10-14, presence of symptoms10,11,15, aortic arch type2,3,7,10,16,17, vessel tortuosity2-4,7,10,13,18 as well as the lesion characteristics themselves2-4,7,10,12,14. The arterial region where the lesion is located and, moreover, the precise delimitation of the arterial segment that will be affected by the action of the stent is still neither standardised nor universally accepted. One of the technical goals of CAS is to achieve a full covering of the carotid plaque by stenting from “normal to normal” arterial regions7,13,19. When planning CAS strategy, it is of paramount importance to know the precise location and the characteristics of this arterial segment because it will interact with materials and techniques during and after the procedure. Traditionally, the evaluation of this segment has mainly considered the lesion characteristics themselves (severity of stenosis, length, calcification, ulceration, irregularity)2-4,7,10,12 but vessel characteristics at the lesion site, such as tortuosity2-4, are less frequently described. This last feature (vessel tortuosity), however, has been mainly related to the distal or proximal vessel anatomy (common carotid artery [CCA] or distal internal carotid artery [ICA], respectively) in many papers7,10,13,18, which have not considered the tortuosity at or close to the lesion site. Vessel tortuosity close to the lesion and likely to be affected during stenting4 should be precisely defined as being part or not part of the vessel segment that will be affected by the stent’s action or pertaining to distal or proximal vessel anatomy. In each scenario, the tortuosity has an impact on different steps of CAS procedure, which requires different materials and strategies.
Another point of discussion when pursuing the anticipation of technical difficulties and risks of the CAS procedure is the fact that there is no single anatomical classification system that can be fully applied for the technical evaluation of the CAS procedure in order to allow for a standardised, sequential and practical analysis of the patient’s angiogram. Propositions for angiographic anatomical classification systems started in 1938, when Fischer published a paper dividing the ICA and its main branches (anterior and middle cerebral arteries) into five segments (C1-C5)20. These segments were based on the angiographic course of the intracranial ICA and were numbered opposite the direction of blood flow. The extracranial ICA was excluded from Fischer’s system. Other anatomical classification systems were proposed for specific stretches of the ICA or for the entire ICA (from the CA bifurcation to the intracranial vessels), but none of them included the CCA21-28 (Figure 1). In clinical settings, these anatomical classification systems are often used by neurosurgeons, neuroradiologists and radiologists to describe cerebral vascular pathologies20-23. With the introduction of CAS in daily medical practice, a new anatomical classification system, which includes the aortic arch anatomy, the CCA and the ICA (from cervical to intracranial) is required to cover all involved technical aspects of CAS.
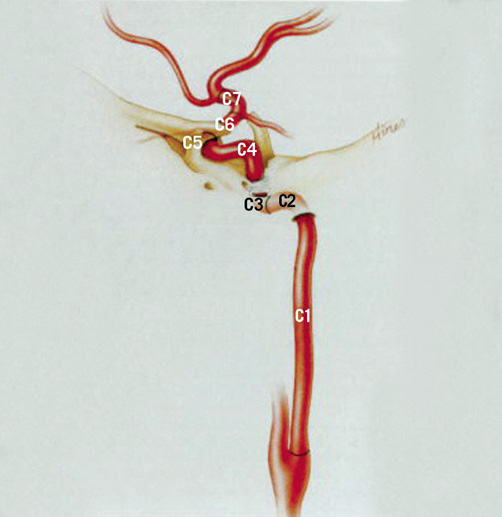
Figure 1 . Classification of the internal carotid artery proposed by Bouthillier et al in 199621 (reprinted with permission from Neurosurgery). The segments of the internal carotid artery are numbered in the direction of the blood flow according to the surrounding anatomy and are labelled as: C1 (cervical); C2 (petrous); C3 (lacerum); C4 (cavernous); C5 (clinoid); C6 (ophthalmic); and C7 (communicating). This classification includes the internal carotid artery (extracranial and intracranial portions) but excludes the common carotid artery.
With the aim of providing a precise delimitation of the arterial segment that will be affected by the stent, the concept of the “stenting segment” for carotid lesions is proposed in this study. The “ABC” classification system for evaluating the precise location of the carotid lesion in the extracranial carotid artery (CA) concerning some vessel landmarks is introduced as well. Finally, a new anatomical-procedural classification system (the “five arterial zones” classification) for delimiting the arterial zones of the CA, which are related to the basic steps of CAS, is also proposed.
Methods
The methodology used in this study to propose new concepts to be applied to the overall carotid area was developed in three different steps and considers: A) a review of the literature via a PubMed search looking for any other anatomical classification systems of the CA that could be applied in the technical evaluation of CAS; B) development of novel concepts regarding the CA anatomy, but related to technical aspects of CAS; and C) testing the applicability of these new concepts in the practical evaluation of CA angiograms as described below.
REVIEW OF THE LITERATURE VIA A PUBMED SEARCH
A review of the literature via a PubMed search with the terms “carotid artery” plus “classification” was carried out in August 2010 to verify the existence of any other classification systems of the CA that could contribute to the technical evaluation of CAS. The search resulted in 801 articles from 1962 up to August 2010. Heterogeneity of terms and delimitations of the arterial segments of the CA were found. No consistent classification system was found that considered all involved anatomical regions for the planning of CAS, i.e., the aortic arch, the entire extracranial CA (CCA and ICA) and the cerebral vessels. This underlined the lack of a logical, sequential and practical scheme for technical evaluation of CAS.
DEFINITION OF NEW CONCEPTS
In this study, new concepts regarding the anatomical division of the extracranial CA were formulated according to the CAS procedural steps. The “stenting segment” concept was elaborated to delimit the arterial region of the CA that would directly be affected by the stent implantation. The “ABC” classification system was chosen for the classification of carotid lesions according to their precise location in the CA. The “five arterial zones” classification system was formulated for the identification of specific arterial zones of the CA, with respect to the basic procedural steps of CAS. These concepts are explained in detail below.
THE “STENTING SEGMENT” DEFINITION
In this study, the “stenting segment” was defined as the straightest arterial segment comprising the carotid lesion and the disease-free arterial regions below and above the carotid lesion, which must be considered for stent implantation, following the concept of stenting from “normal to normal” (Figure 2). In some cases, a short “non-diseased region” after the carotid lesion could be chosen to avoid including a vessel curve. It was established that five millimetres would be the shortest length of the disease-free arterial region (above the carotid lesion) because this minimum length was thought to be the shortest segment required for a well-trained operator to achieve a successful stent implantation. When the vessel curve was located at a distance shorter than that, it was necessarily considered as part of the “stenting segment”. When the vessel curve, proximally or distally to the lesion, was not comprised by the “stenting segment”, it was necessarily classified as proximal or distal vessel tortuosity, respectively.
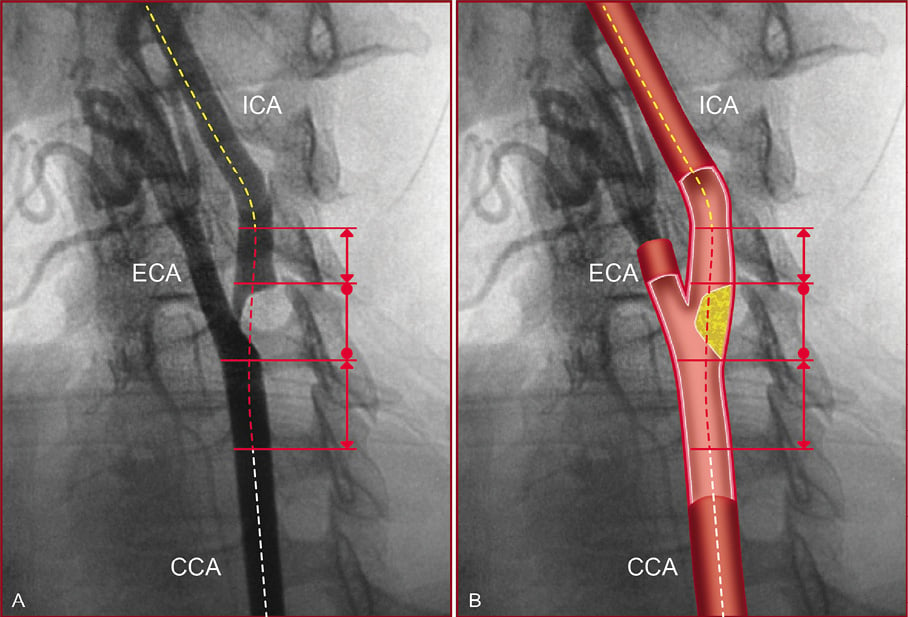
Figure 2. The “stenting segment” scheme is represented on a conventional angiography (A), and on an illustration of the same angiogram (B). The “stenting segment” (red dotted line) comprises the lesion region (limits are represented by the solid line with dots), and the disease-free arterial regions (limits are represented by the solid line with double arrows) below and above the carotid lesion that must be considered for stent implantation. Proximal vessel anatomy (white dotted line) and distal vessel anatomy (yellow dotted line). ICA: internal carotid artery; ECA: external carotid artery; CCA: common carotid artery
THE “ABC” CLASSIFICATION SYSTEM FOR CAROTID LESION LOCATION
In this study, the obstructive lesions of the extracranial CA were “universally” classified according to the relation between the “stenting segment” limits and the CA bifurcation level. Mnemonically, the carotid lesions (the brachiocephalic trunk [BCT] lesions were also included in this study) were classified according to the direction of the arterial blood flow in types A, B or C, depending on whether the “stenting segment” limits were below, at or above the bifurcation level, respectively. The precise location of the carotid lesions were also determined by the subdivision of the ABC levels as described in detail in Figure 3.
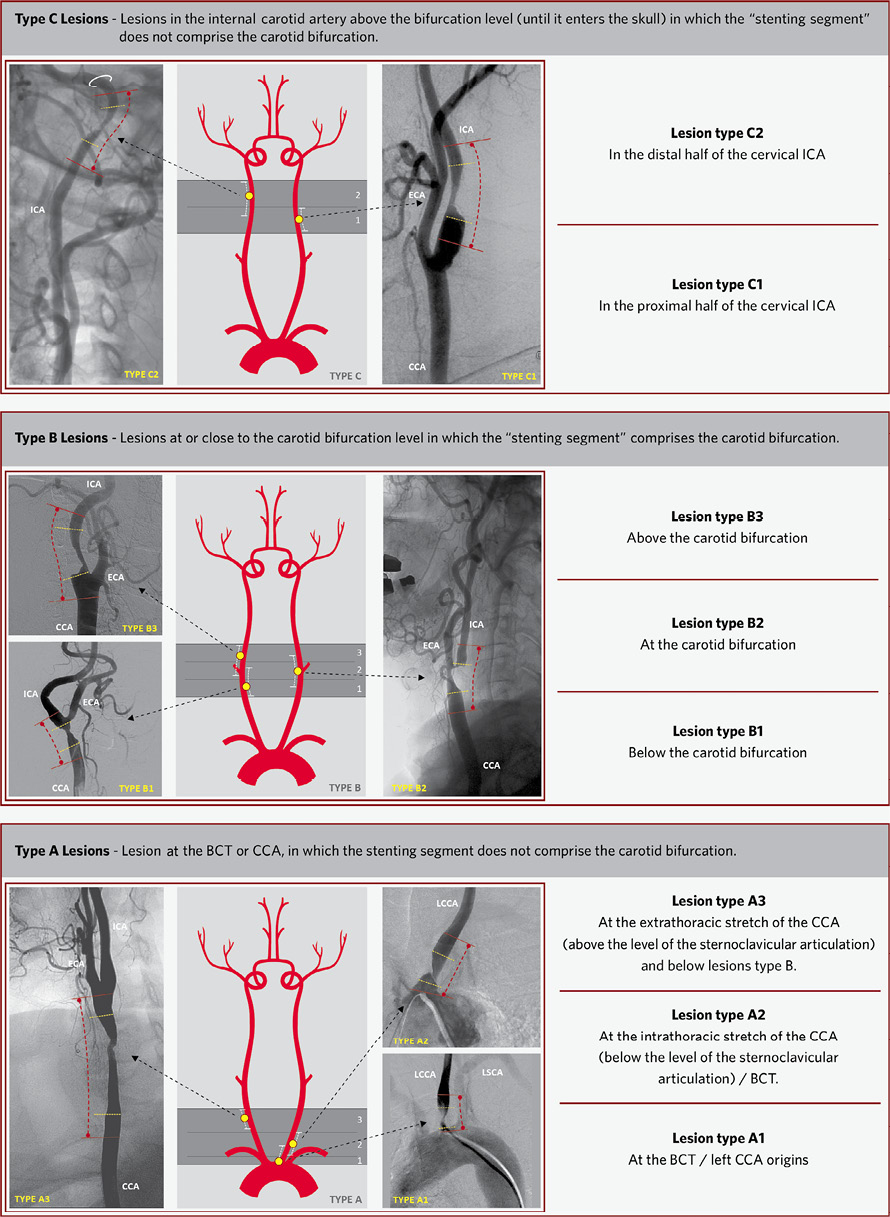
Figure 3. The “ABC” mnemonic classification system for extracranial carotid lesion location. The extracranial carotid lesions were classified into type A (bottom drawing), type B (centre drawing) and type C (top drawing) according to the relation between the “stenting segment” and the carotid artery bifurcation levels. The subdivision of the lesions A (A1, A2 and A3), B (B1, B2 and B3) and C (C1 and C2) are explained in detail in each box and are illustrated on the angiograms parallel to the central drawings. In the central drawings are illustrated: the lesion (yellow dot) and the “stenting segment” limits (white lines). On the carotid artery angiograms are illustrated: the limits of the carotid lesions (yellow lines), the “stenting segment” limits (red lines). The level of the carotid foramen is represented by the white circle in the C2 lesion. CCA: common carotid artery; ICA: internal carotid artery; ECA: external carotid artery; LCCA: left common carotid artery; LSCA: left subclavian artery
THE “FIVE ARTERIAL ZONES” CLASSIFICATION SYSTEM
In this study, the extracranial CA of every individual angiogram was divided into three arterial zones relating to the basic procedural steps of CAS. After delimiting the “stenting segment”, the extracranial CA was divided into: the “proximal vessel zone” (from the origin of left CCA or BCT to the “stenting segment”); the “stenting segment” zone itself and; the “distal vessel zone” (considering the CA above the “stenting segment” until it enters the skull through the carotid foramen). The “five arterial zones” classification system is composed of these three arterial zones added to the two classic arterial regions –the aortic arch and cerebral vessel anatomies (the CA from the carotid canal to its branches), which are herein called “aortic arch” and “cerebral vessels” zones, respectively. Figure 4 shows the schematic application of the “five arterial zones” classification system to the different types of lesion location (lesion types A, B and C according to the “ABC” system).
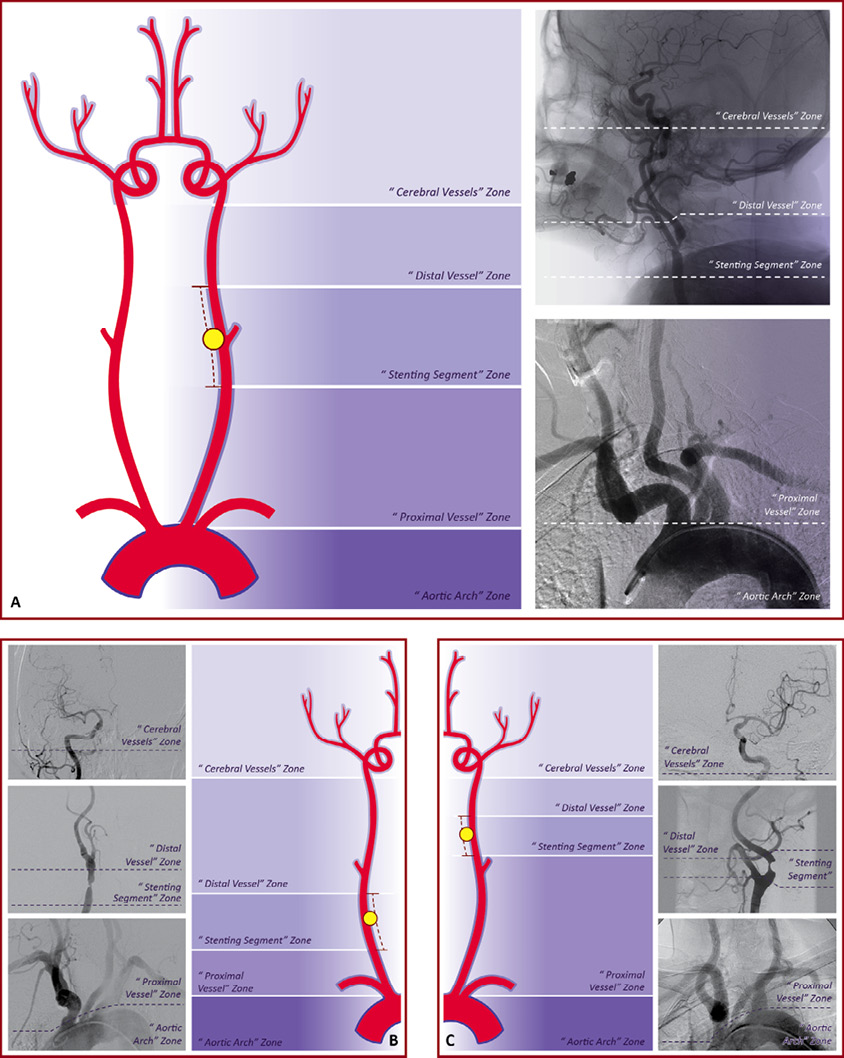
Figure 4. Schematic application of the “five arterial zones” classification system. The classification system is schematically applied to each lesion type (A, B and C). In Figure A, the lesion type B2 is represented in the left drawing and in a practical evaluation of a carotid angiogram. In Figure B, the lesion type A3 is represented in the right drawing and in a practical evaluation of a carotid angiogram. In Figure C, the lesion type C1 is represented in the drawing and in a practical evaluation of a carotid angiogram. In the drawings, the carotid lesions are represented by the yellow ball and the “stenting segment” limits by the red dashes. On the carotid angiograms, the white dashes delimit the level between each arterial zone.
TESTING THE APPLICABILITY OF THE NEW CONCEPTS
The “stenting segment” concept, the “ABC” and the “five arterial zones” classification systems were retrospectively tested by the authors on a total of 250 consecutive CA angiograms in which an obstructive disease (atherosclerotic, restenotic [post-CAS or post-CEA] or fibromuscular dysplasia) was identified. All carotid angiograms included a study of intracranial circulation. Chronic total occlusions, tandem lesions and other vascular pathologies such as arterial aneurysm and arterial dissection were not included in this study. Patients who had carotid lesions with the “string sign” were also included, but the “distal vessel zone” characteristics were previously known as “unpredictable”. Patients with bilateral carotid lesions were counted twice because each CA was evaluated individually. Applicability in delimiting the “stenting segment” limits according to the “stenting segment” definition, classifying the carotid lesion according to the “ABC” classification system definitions and identifying arterial zones according to the “five arterial zones” classification system definitions were tested by the authors on all studied angiograms.
Results
From April 2009 to May 2010, 232 patients had their carotid angiographic studies evaluated. In 18 patients, bilateral carotid lesions were identified. A total of 250 CA angiograms were evaluated.
APPLICABILITY OF THE “STENTING SEGMENT”
The delimitation of the “stenting segment” limits according to the definitions of this study was achieved in all (100%) of the studied carotid angiograms (Table 1). In patients with ostial lesions of the left CCA (two cases) and ostial lesion of the BCT (one case), the “normal region” of the “stenting segment” below the carotid lesion was previously known as non-existent. Even in these three cases (1.2%), the delimitation of the “lesion region” and the “normal region” above the lesion was successfully achieved.
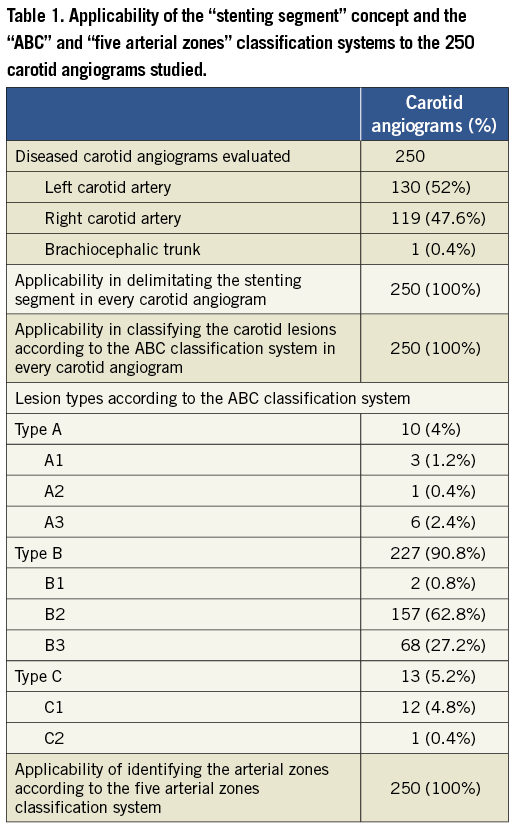
APPLICABILITY OF THE “ABC” CLASSIFICATION SYSTEM
The relationship between the “stenting segment” and the CA bifurcation level was successfully identified in all studied CA angiograms allowing the lesions to be classified into types A, B or C in all (100%) cases (Table 1). All lesions were also successfully classified into the subtypes A1, A2, A3, B1, B2, B3, C1 and C2. The precise delimitation between lesion types A2 and A3 (the intra-thoracic and extra-thoracic stretches of the CA, respectively) was better achieved when the antero-posterior (AP) projection was available, where the sternoclavicular articulation (landmark for delimiting the intra- and extra-thoracic limits29) and the lesion levels were easily seen. The vast majority of the lesions were classified as type B2 (62.8% of the lesions) or B3 (27.2% of the lesions), which together accounted for 90% of the carotid lesions.
APPLICABILITY OF THE “FIVE ARTERIAL ZONES” CLASSIFICATION SYSTEM
The applicability of the “five arterial zones” classification system was confirmed in all (100%) cases (Table 1). The delimitation of the aortic arch and cerebral vessel anatomies according to the traditional anatomic definition were intuitive in all cases. The delimitation of the “stenting segment” zone, the “distal vessel” zone and the “proximal vessel” zone was fully achieved in 246 (98.4%) of the carotid angiograms. In patients with ostial lesions of the left CCA (two cases), ostial lesion of the BCT (one case), or a very proximal lesion of the left CCA, which allowed the delimitation of the stenting segment only (one case), the proximal vessel zone was previously known as non-existent (a total of four cases; 1.6%). Even in these cases, the delimitation of the “stenting segment” and the “distal vessel” zones was successfully achieved.
Discussion
The decision to include the BCT lesions in a CAS classification was taken due to the fact that BCT lesions and lesions at the proximal part of the left CCA share many technical aspects, especially when considering aorto-ostial lesions which require a precise deployment of the carotid stents while dealing with poor guiding catheter (GC) stability. In cases of aorto-ostial lesions (BCT and left CCA), the “normal region” of the “stenting segment” and the “proximal vessel” zone do not exist because of the lesion location itself, which is reflected in the actual CAS procedure by the common practice of allowing the stent to protrude 5 mm inside the aorta lumen to cover the entire vessel ostium. The main differences between the BCT and proximal left CCA are the greater diameters of BCT stents and the possibility of dealing with bifurcation lesions (right subclavian and right CCA).
The ostial lesions of the right CCA (classified as type A2 lesions) were not considered to be the same as the lesions at the ostium of the left CCA (classified as A1 lesions) because they are not aorto-ostial lesions, and therefore do benefit from the BCT support to the GC/LS, which is, in our view, much more important when analysing the basic steps of CAS.
The ABC classification system was created from the practical evaluation of carotid angiograms and we postulate the theoretical importance of classifying the precise location of the carotid lesion based on the fact that, for each lesion type, diverse material and techniques are required. The vast majority of lesions found in this study were type B lesions (90.8%), probably reflecting the daily practice scenario when performing CAS, but not infrequently we were also faced with the technical discussion of lesion types A and type C, which accounted for 9.2% of all evaluated patients. In our opinion, the technical discussion of type A1 lesions should consider the use of balloon-expandable stents due to both the requirement for precise stent deployment and the higher radial force required by the greater resistance of ostial lesions. In type A2 lesions, balloon-expandable stents could be considered, except in those cases in which the “stenting segment” extends into the extra-thoracic stretch of the CA, where self-expandable stents are definitely required due to the risk of stent compression4,30-33. In the technical discussion of A1 lesions, the use of proximal embolic protection devices (EPD) was considered impracticable, and even in types A2 or A3 lesions were considered very risky. In these cases we considered that only the use of the balloon sheath of the NPS® proximal EPD (W. L. Gore & Associates, Inc., Medical Products Division, Flagstaff, AZ, USA) was permissible, the MO.MA® system (Medtronic/Invatec S.p.A., Roncadelle, Italy) would not be allowed in any type A lesions. When discussing type B lesions, the use of self-expandable stents should be considered mandatory due to the risk of stent compression when using balloon-expandable stents4,30-33. Type B1 lesions were thought to make the use of proximal EPD risky, because the system has to cross the lesion when accessing the external CA (behaving similarly to type A3 lesions when choosing the EPD). In type B2 and B3 lesions, both proximal and distal EPD could be chosen. In type C, the use of self-expandable stents is mandatory due to the risk of external compression4,30-33. A distal EPD would face difficulty in C1 lesions due to the short landing zone for the distal EPD and would be a problem in C2 lesions because the distal vessel stretch continues into the skull (with many vessel curves). The ABC classification system had its applicability confirmed in all cases in this study and, due to its intuitive use, could be of help during the practical evaluation of carotid lesions when considering performing CAS.
Figure 5 summarises the authors’ perception of the potential impact of each arterial zone of the “five arterial zones” classification system on the planning of the basic steps of CAS and on the choice of the material characteristics. Because these arterial zones are in fact directly related to a specific step of CAS, their evaluation before the procedure could help the operator to anticipate technical difficulties and risks, helping to choose the most appropriate material and technique for a specific patient with a specific carotid anatomy and lesion.
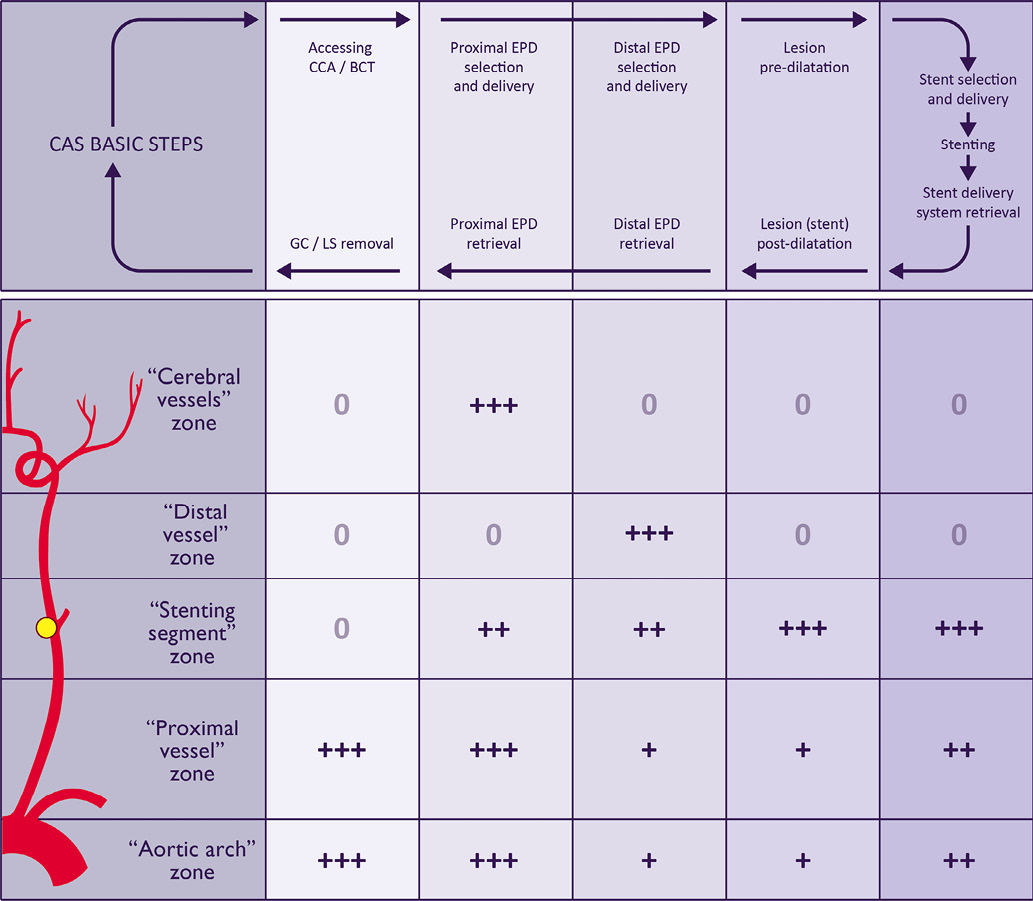
Figure 5. Theoretical impact of the “five arterial zones” classification system on the technical evaluation of the basic CAS steps according to the authors’ perception. The technical evaluation of CAS was divided into: 1) accessing and stabilising the system (guiding catheter, long-sheath or proximal EPD) in the CCA/BCT; 2) selection, deployment and retrieval of proximal EPD; 3) selection, deployment and retrieval of distal EPD; 4) lesion pre- and post-dilatation and; 5) stent selection, delivery and deployment. 0: no influence; +: little influence; ++: moderate influence; +++: strong influence; CAS: carotid artery stenting; CCA: common carotid artery; BCT: brachiocephalic trunk; EPD: embolic protection devices; GC: guiding catheter; LS: long-sheath
The “aortic arch” zone (the traditional “aortic arch”) characteristics have been described as impacting on the catheterisation process of the supra-aortic trunks and on the stabilisation of the system (GC/LS)3,4,16,17. A type III aortic arch would require a more flexible GC to deal with vessel tortuosity. Higher deliverability devices (such as braided mesh and open cell stents) reduce the “stress” on the system during the advance through the aortic arch level, which tends to minimise the risks of prolapsing the system into the aorta. These concerns would be equally applicable in the evaluation of the “proximal vessel” zone. Distal protection devices would not be a matter of major concern when approaching tortuous “aortic arch” and “proximal vessel” zones. LS or proximal EPD tends to “push up” the vessel tortuosity, which could distort the normal anatomy or accentuate the “stenting segment” and “distal vessel” zone tortuosity.
The “stenting segment” zone characteristics would promote technical discussion mainly when choosing stent characteristics, cerebral protection devices and the need for lesion predilatation. The “stenting segment” zone analysis includes the lesion and vessel characteristics. The vessel characteristics should also include the “stenting segment” tortuosity (composed of the take-off angle of the ICA in type B lesions and of any other angle of the segment, regardless of whether it includes the carotid lesion region or whether it lies in the “normal arterial region”). A general perception is that arterial tortuosity would be better approached with flexible stents; high emboligenic plaques would be better approached with high scaffolding stents (braided mesh and nitinol closed-cell stents); large CCA would be better approached with larger stents; and larger vessel mismatch would be better approached with conic stents. In cases of critical carotid lesions, the passage of filters could carry the potential risk of plaque embolisation and would be better handled by proximal EPD. The lesion predilatation should be strongly considered in this lesion subset. The use of distal EPD would be a point of caution in cases of angulated “stenting segment” zone, not only when deploying the device but during its retrieval because of the risk of filter entrapment in the stent cells.
The “distal vessel” zone technical analysis has the theoretical benefit of anticipating technical difficulties when using distal EPD, as well as in cases requiring neurological rescue manoeuvres (when cerebral embolisation occurs)2. Filters should be avoided in very tortuous and diseased “distal vessel” zones (which make difficult the delivery and retrieval of distal EPD). These situations would be better approached with the use of a proximal EPD.
The “cerebral vessel” zone evaluation normally impacts the use of proximal EPD because of the risk of cerebral intolerance during the endovascular clamping of the carotid flow. Its analysis should include: evaluating the intracranial arterial pattern; verifying the existence of any vessel diseases (stenosis, aneurysm, arteriovenous malformations and others); identifying the cerebral collateral flow by checking the integrity of the circle of Willis by studying the patency of the anterior and, if necessary, the posterior communicating arteries to establish hemisphere dominance and to identify cases of isolated hemisphere3,4,7. Filters should be strongly encouraged in cases of isolated hemisphere.
Finally, we may postulate that the delimitation of the “stenting segment” may vary according to operator experience (and here we are not considering the difficulties in acquiring all the available lengths of carotid stents, which would obviously directly influence the “stenting segment” length). Even in these cases, the “five arterial zones” classification would follow exactly what the operator had concretely defined as “stenting segment” limits. If the “stenting segment” limits of a beginner operator include a vessel tortuosity (which could be avoided by an expert operator) and the technical aspects of this tortuosity are taken into consideration (choosing flexible stents, considering proximal EPD) using this method, then the goal of this classification of being inserted in the real world of CAS would be achieved. Conversely, differences in “stenting segment” limits would not be a concern during the standardisation of data to be applied in clinical research because the level of experience of the operators involved in these trials would probably not be less than that of expert operators.
To our knowledge, the “five arterial zones” classification system is the first system to be proposed to standardise the technical evaluation of CAS.
Conclusion
The “stenting segment” concept along with the “ABC” and “five arterial zones” anatomical-procedural classification systems are applicable in patients with extracranial CA obstructive disease.
Conflict of interest statement
The authors have no conflicts of interest to declare.

