Abstract
Aims: It has been stated that experienced physicians with a high volume of carotid artery stent (CAS) procedures have low complication rates, including cerebrovascular accidents (CVA). Complication rates, however, are not known for physicians with a low volume of CAS (<50 CAS/yr) but with a high volume of other peripheral endovascular procedures. Since the techniques used in CAS are similar to those used routinely in other peripheral interventions, we hypothesise that high volume peripheral interventional operators with appropriate training would have low complication rates during CAS procedures.
Methods and results: We reviewed all CAS procedures that were performed from 2004-2009 by an experienced physician with a high peripheral interventional volume (>250 interventions/year). Filter-based embolic protection devices were used during each CAS procedure. Each patient was followed for 30 days and data on major adverse cardiac and cerebral events (MACCE) collected. Ninety-two patients with ninety-five lesions were treated with CAS. Recent CVA was the indication in half of the patients and asymptomatic high-grade stenosis was the indication in the other half. Twenty-one (23%) patients had previous history of CEA and six (7%) patients had previous CAS in the contralateral side. All CAS procedures were technically successful. One patient (1.1%) had a TIA with total resolution of symptoms in ten minutes. There were no major strokes. MACCE rate was 1.1% at 30 days.
Conclusions: We found a very low complication rate following CAS with embolic protection performed by an experienced physician who has a relatively low CAS volume but a high volume of other peripheral interventions.
Abbreviations
CAS: carotid artery stenting
CVA: cerebrovascular accident
CEA: carotid endarterectomy
PVD: peripheral vascular disease
CAD: coronary artery disease
DM: diabetes mellitus
HTN: hypertension
MACCE: major adverse cardiac and cerebral event
CMS: Centers for Medicare and Medicaid Services
ACT: activated clotting time
Introduction
Carotid artery stenosis has historically been treated with carotid endarterectomy (CEA). Carotid artery stenting (CAS) is rapidly becoming an acceptable alternative option for carotid artery stenosis, especially in those patients that are at high risk for surgery1,2. CAS provides a percutaneous approach to treat carotid artery stenosis with an acceptable complication rate. Guidelines on operator qualifying credentials and training are evolving3-5. There is evidence to support that with increased physician experience, technical success increases and complication rates decrease7-10. It is also stated that high volume CAS operators (>50 CAS procedures/year) would have a low complication rate of cerebrovascular accidents (CVA)7,9. The complication rate of low volume CAS operators (< 50 CAS procedures/year) who have a high volume of peripheral interventional procedures (>250 peripheral interventions/year) is not known. CAS and peripheral interventional techniques are complimentary, including experience with catheter and guidewire manipulation, deployment of embolic protection devices, percutaneous transluminal angioplasty as well as stenting techniques and management of anticoagulation during the procedure2,3. We, therefore, hypothesised that an experienced physician who is trained in CAS but with a low volume of CAS procedures with a high peripheral interventional volume would have a low cerebrovascular complication rate.
Methods
We performed a retrospective cohort study and reviewed all CAS procedures performed from 2004 - 2009 by an operator with high peripheral interventional volume (>250 interventions/year). Data was collected by chart and electronic record review. All angiograms were reviewed by two operators. Data on demographics, procedure variables and complications were collected on each patient. All CAS procedures were performed with adjunctive distal embolic protection devices. Technical success was defined as a greater than 50% decrease in carotid artery stenosis with successful retrieval of the protection device. Clinical success was defined as a technical success to 30 days without incident of a major adverse cardiac or cerebrovascular event (MACCE). Recent history of TIA or CVA was defined as having occurred in the last six months.
Procedure details
All patients were pre-medicated with aspirin and clopidogrel. Antihypertensive medications were held back on the day of the procedure. Intravenous heparin was used in all patients and a target ACT of >250 seconds was achieved. All patients had aortic arch, bilateral carotid angiograms and intracranial angiograms performed using a 4 Fr system prior to the CAS procedure. Bilateral carotid angiograms are routinely performed at our institution prior to CAS. All patients have one or more non-invasive imaging modalities performed before the angiograms which included carotid duplex scans (most commonly) and magnetic resonance angiograms. Intracerebral angiograms of the side treated were performed before and after CAS. The common carotid artery was engaged with a 6 Fr sheath, the internal carotid artery lesion was crossed with 0.014” guidewire followed by placement of a distal embolic protection device. SpiderFX® (EV3 Endovascular, Inc., Plymouth, MN, USA) and Emboshield™ (Abbot Corporation, Abbot Park, IL, USA) were the most common embolic protection devices used. All lesions underwent pre-dilatation with a semi-compliant balloon (3.0 or 3.5 mm in diameter and 20 mm in length). This was followed by placement of an appropriately sized self-expanding stent. Xact® Carotid stent system (Abbot Corporation, Abbot Park, IL, USA) and Protégé RX® Carotid stent system (EV3 Endovascular, Inc., Plymouth, MN, USA) were the most common stents used. Xact® Carotid stent system is a closed cell stent architecture while Protégé RX® is an open cell design carotid stent system. Post-dilatation was performed using a 5.0 mm or 5.5 mm diameter by 20 mm long balloon. The protection device was then retrieved. Post-CAS angiograms were performed. Frequent neurological exams were performed both periprocedurally as well as in the ICU, where all patients stayed for the night.
Statistical methods
Continuous variables are expressed as mean ± standard deviation and categorical variables as values and percentages. Continuous variables were compared using the student t-test. Comparison of categorical variables was done by chi-square test or the Fisher exact test. A p value of <0.05 was considered statistically significant. All statistical analysis was performed on statistical package for the social sciences (SPSS) for Windows (version 16.0, SPSS Inc., Chicago, IL, USA).
Results
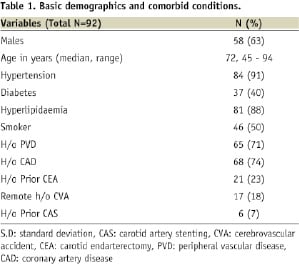
Ninety-two patients and ninety-five lesions were treated with CAS (three patients had staged bilateral CAS). Median age was 72 years with males comprising 63% of the cohort. Octogenarians constituted twenty-six percent of the patients undergoing CAS in our study (n=24).
Previous history of DM, HTN, tobacco abuse, dyslipidaemia and CAD was present in 40%, 91%, 50%, 88% and 74% of patients respectively. Two-thirds of the patients had previous history of documented peripheral vascular disease. Eighteen percent of the patients had a remote history of a stroke. Twenty-one (23%) patients had previous history of CEA and six (7%) patients had previous CAS (Table 1).
Technical success was achieved in all CAS procedures. There was no periprocedural lesion or other vascular complications.
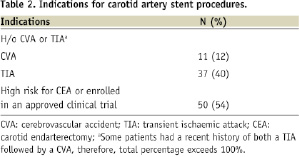
Recent CVA was the indication in half of the patients. Eleven patients had recent history of ipsilateral stroke and a further thirty-seven patients had a recent history of transient ischaemic attack. The rest of the CAS procedures were performed in patients with asymptomatic high-grade stenosis (defined by stenosis severity of ≥80%). These patients were either at high risk for CEA and/or enrolled in approved investigational clinical trials intended to validate the safety and efficacy of CAS with an embolic protection device and a particular self-expanding stent. High risk for CEA was defined by SAPPHIRE criteria1. Mean filter time was 9±2 minutes. One patient (1.1%) had a TIA with total resolution of symptoms in ten minutes. There were no major strokes. The 30 day MACCE rate was 1.1%.
During these consecutive cases, six patients were referred for CEA after having a diagnostic carotid angiogram. All of these were for unfavorable anatomy for CAS (four patients for complex aortic arch and two patients for heavy calcification at the lesion site).
Discussion
Studies suggest that the incidence of cerebrovascular complications during carotid artery stenting is lower for high volume CAS operators than low volume CAS operators7-10. It is also reported that the risk of complications decreases as operator experience increases7-9. A number of medical and surgical subspecialties perform CAS including interventional cardiologists, vascular surgeons and interventional neuroradiologists. Societies representing each of these subspecialties are working on training and credential guidelines3-5. It is interesting to note that there is marked variation in these guidelines, with unified guidelines yet to be published. However, having different skill subsets especially in the use of guiding catheters, vascular access sheaths, distal protection devices and guidewires in the treatment of endovascular disease varies according to the subspecialty and this may preclude a unified training guideline3,4,6.
Due to the current CMS restrictions and insurance reimbursement stipulations, many qualified endovascular physicians with significant technical expertise, accrued practice knowledge and skills to perform CAS safely are restricted from performing a large number of CAS procedures (>50/year).
Besides having the technical expertise in doing endovascular procedures, the interventionalist who performed the CAS procedures reviewed in this paper had gone through didactic and proctored training programs for carotid/cerebral angiography and CAS. It will be interesting to see in the post-market surveillance registries the impact of peripheral procedure volume and experience on the success of carotid interventions.
In addition to the operator training and expertise at performing peripheral vascular interventions, other contributing factors for a low complication rate include careful CAS patient selection, periprocedural patient medical management and diligent use of the selected distal embolic protection system with coordinated efforts to minimise filter deployment times. During this study, only six (7%) patients were deemed not suitable for CAS due to anatomic factors identified during baseline arch angiography11. All procedures were performed with the filter deployment times less than 12 minutes, which we think contributes favourably in reducing complications.
The widespread acceptance of CAS as a treatment option for patients with carotid artery disease requires evidence-based data showing that CAS has a procedural low complication rate comparable to that for CEA12,13. Besides proper didactic training, different societies must recognise the strengths and weaknesses of physicians with different skill subset3,4,6. We have shown in this paper that a physician, well disciplined in the practice of peripheral vascular intervention, can successfully perform protected CAS in symptomatic and asymptomatic patients and achieve an acceptable low procedure-related in-hospital complication rate for those patients.
All guidelines are based on proctored CAS procedures. None of the guidelines take into account the level of peripheral interventional skills. Interventional skills required in CAS are similar to those required in peripheral interventional procedures including the use of embolic protection devices. Our data suggest that physicians with a high volume of peripheral interventional procedures will have a low complication rate in CAS procedures. To our knowledge, this is the first paper reporting this particular finding.
Limitations
Limitations include the fact that this is a retrospective cohort analysis and a single operator experience. This study should be considered as preliminary data that needs to be studied prospectively in a controlled fashion across multiple operators belonging to different subspecialties from different centres.
Conclusion
We found a very low CAS complication rate for an operator who has a relatively low annual volume for CAS, but high volume of other peripheral interventions. To our knowledge, this is the first study looking at an important aspect of CAS training and credentialing.

