Abstract
Aims: This prospective registry study was intended to evaluate outcomes and predictors of adverse events following carotid artery stenting (CAS).
Methods and results: Patients received neurological and duplex exams before CAS, prior to discharge and at 30- and 180-day follow-up. Multiple regression analysis included patient- and procedure-related characteristics. The MACCE endpoint comprised stroke, myocardial infarction and death. Three hundred and seventy-five consecutive patients underwent CAS between 1998 and 2011. Mean age was 69±9.1 years; 53% were symptomatic within the preceding six months. Mean time to CAS was 23 days in patients with TIA and 31 days with stroke (p=0.029). The MACCE rate was 1.6% during intervention and 4.0%, 5.6% and 5.9% at discharge, day 30 and day 180, respectively. TIA occurred in 31 cases (9.6%) within 30 days. A history of TIA was independently associated with MACCE (OR: 2.88; p=0.04). Furthermore, a history of hyperlipidaemia (OR: 4.02, p=0.029), MI (OR: 2.93, p=0.007) and age ≥70 (OR: 1.89, p=0.033) were independent predictors for the combined endpoint MACCE plus TIA.
Conclusions: TIA is an underappreciated adverse event following CAS. Pre-procedural TIA was an independent risk factor for adverse outcomes, while stroke was not, probably related to the timing of the procedure relative to the index event.
Abbreviations
CAS: carotid artery stenting
CREST: Carotid Revascularization Endarterectomy versus Stenting Trial
MACCE: major adverse cardiac and cerebrovascular events
NASCET: North American Society of Carotid Endarterectomy Trial
SAPPHIRE: Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy
SPACE: Stent-Protected Angioplasty versus Carotid Endarterectomy
TIA: transient ischaemic attack
Introduction
Carotid artery stenting (CAS) for atherosclerotic stenosis remains a controversial intervention and it is uncertain which patients are most likely to benefit1. Individual decision making in patients with carotid artery stenosis is challenging, as clinicians must attempt to translate the results of randomised controlled trials, including selected patient cohorts, to individual patients2-6. The careful selection of interventionalists and the narrow inclusion and exclusion criteria of randomised trials can lead to uncertain generalisability to clinical practice. On the other hand, registry studies deliver advantages with insights into real-life populations and routinely applied operating procedures. Importantly, recently published randomised trials were designed many years ago, partially applying selection criteria that do not conform to contemporary guidelines and endovascular protocols, excluding endpoints such as transient ischaemic attacks (TIA) and myocardial infarction (MI)2,7,8.
This study prospectively evaluated consecutive patients undergoing CAS utilising systematic neurological and cardiac assessments (Figure 1). We intended to search for independent predictors of major adverse cardiac and cerebrovascular events (MACCE) following CAS.
Methods
ENROLMENT OF PATIENTS AND DATA COLLECTION
Consecutive patients with indications for CAS at the Department of Cardiology and Angiology II, University Heart Center Freiburg, Bad Krozingen, were prospectively included from July 1998 to April 2011. Informed consent was given for the procedure and for prospective data acquisition. The decision to proceed with CAS was determined by a neurologist taking into consideration published guidelines9,10. The main inclusion criteria of patients were discrete stenoses of the internal carotid artery of ≥50% in symptomatic and ≥70% in asymptomatic patients by angiography and expected ability to deliver the stent. Patients with intolerance to any antiplatelet medication, bleeding diathesis, evolving strokes, and large strokes at risk of haemorrhagic conversion were excluded. Data on clinical assessment, duplex ultrasonography (DUS) and angiography were prospectively collected. Pre- and post-procedural neurological examinations were performed by a consultant neurologist. Patients were classified as symptomatic if a TIA, amaurosis fugax or stroke ipsilateral to the carotid stenosis had occurred in the past six months prior to admission. TIA was defined as a focal neurological deficit lasting no more than 24 hours. The modified Rankin Scale (mRS) was used to assess the severity of pre-interventional stroke. The study was in conformity with the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/17c.pdf) and ISH-GCP guidelines (http://www.ich.org). Approval was granted by the local ethics committee. Quality assurance was by CoreLab Bad Krozingen GmbH, Bad Krozingen, Germany.
STANDARDS OF PATIENT EVALUATION AND DATA PROCESSING
The NASCET-defined degree of internal carotid artery stenosis was determined by experienced sonographers using established classification criteria after internal validation11. Duplex equipment included ATL 5000 and iU22 (Philips Medical Systems, Eindhoven, The Netherlands). Angiography was performed with Hicor Axiom equipment (Siemens AG, Munich, Germany) immediately before CAS. All DUS images and angiographic cine runs were blinded and analysed at CoreLab Bad Krozingen, using quantitative vascular analysis software (QAngio XA 7.1; Medis, Leiden, The Netherlands).
PERI- AND POST-PROCEDURAL MEDICATION
Patients received dual antiplatelet therapy at least 48 hours before CAS, consisting of a loading dose of 300 mg clopidogrel (600 mg after 2003) in addition to 500 mg aspirin. Clopidogrel 75 mg once daily or, in case of clopidogrel intolerance, ticlopidine 250 mg twice daily was given for four weeks after CAS, and then patients were switched to aspirin 100 mg daily. Medication adherence was queried at each follow-up visit. One milligram of atropine was administered before stent deployment. The prescription of statins and antihypertensive drugs was according to the recommendations of the National Cholesterol Education Program Adult Treatment Panel and the Joint National Committee, individually adjusted to risk profiles (http://www.nhlbi.nih.gov/guidelines/index.htm).
STENTING AND PERI-INTERVENTIONAL EXAMINATIONS
Procedures were uniformly carried out by a single, very experienced operator, routinely attempting to perform stenting after placement of distal filter protection devices. Post-dilation was carried out in all patients. Baseline and final extracranial and intracranial angiograms were made in the same projections. An intervention was defined as successful according to SPACE criteria3. Patients were monitored for neurological and cardiovascular symptoms during the intervention. Cardiac biomarkers and electrocardiograms were analysed six to 24 hours before and after CAS. MI was defined by enzyme abnormalities plus symptoms or ST-segment changes2. In addition, neurological examinations were carried out every three hours for at least 24 hours after the intervention. Prior to discharge, a final neurological examination was performed by a neurologist.
FOLLOW-UP ASSESSMENTS AND ENDPOINTS
Patient follow-up was scheduled at day 30±5 and at day 180±21 (Figure 1). Each visit consisted of a neurological exam, a cardiovascular exam and a DUS study of the carotid arteries.
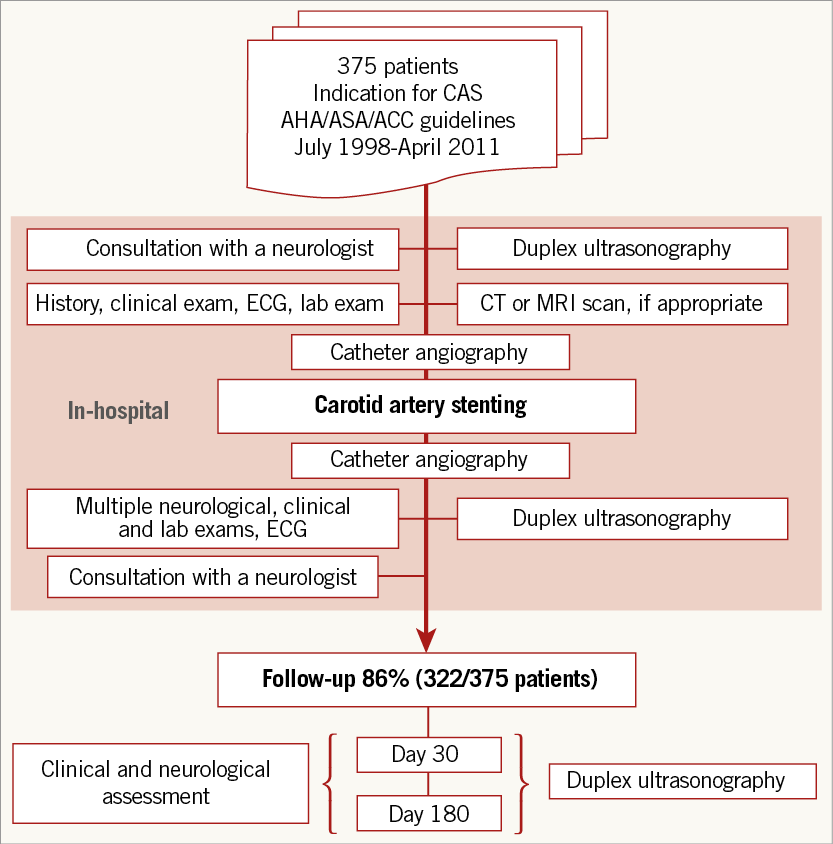
Figure 1. Study design. CAS: carotid artery stenting; CT: computed tomography; ECG: electrocardiogram; MRI: magnetic resonance imaging
Neurological events were documented and categorised as TIA (a focal neurological deficit lasting no more than 24 hours), as a minor stroke (with duration longer than 24 hours but with regression within seven days), or as a major stroke (with a focal neurological deficit persisting more than seven days). MACCE consisted of the occurrence of stroke, MI and death. We also included ipsilateral TIA within a composite endpoint MACCE+T, previously not analysed in randomised controlled trials.
STATISTICAL ANALYSIS
SPSS, Version 18.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. Significance was assumed at p<0.05. Univariate analysis was performed including patient-related variables (Table 1) by employing Fisher’s exact test or the chi-square test and logistic regression analysis, as appropriate. Risk factors identified in univariate analysis at the p<0.1 level and outcome predictors established in other trials were included in the multivariate logistic regression analysis. Two-tailed Mann-Whitney U statistics were used to test for significance of numeric differences between independent samples. Continuous data are expressed as mean±SD.
Results
PATIENT POPULATION AND BASELINE CHARACTERISTICS
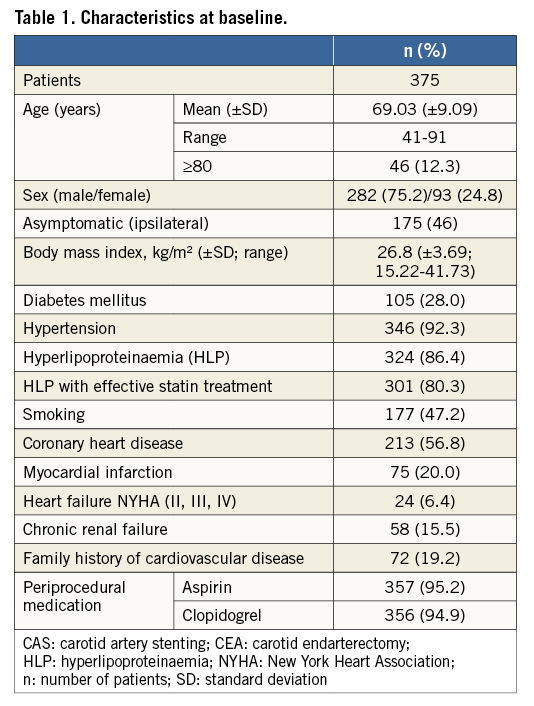
Three hundred and seventy-five consecutive patients with carotid artery stenosis underwent CAS; 282 (75.2%) were men, and the mean age was 69±9.1 years. Patient characteristics at baseline are shown in detail in Table 1. The carotid stenosis was related to a neurologic event in 53% of cases (20 minor, 40 major strokes, 89 TIA including 51 amaurosis fugax). Prior to CAS, an mRS ≥3 was documented in 19 cases (5.1%). Prior to the intervention, high-grade carotid artery stenoses (≥70%) were determined by DUS in 89% of cases (n=334), and 50-69% stenoses in 11% (n=41). Contralateral occlusion was found in 12% of cases (n=44). In 22 patients CAS was performed as a reintervention after either carotid endarterectomy (CEA, n=13, 3.5%) and/or CAS (n=9, 2.4%). According to the risk classification of the “Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy” (SAPPHIRE) trial, A) 204 (54.4%) of our patients had no high-risk features as defined by SAPPHIRE criteria, B) 94 (25.1%) had at least one risk factor as defined by the SAPPHIRE inclusion criteria (e.g., contralateral occlusion or age >80), and C) 77 (20.5%) were considered to be at a very high risk for negative outcomes and consequently they would have been excluded from SAPPHIRE, e.g., having developed a stroke within the previous 48 hours or given the presence of an intraluminal thrombus12. The mean time to treatment in patients who suffered a neurologic event up to 180 days prior to intervention was 31±30 days for stroke versus 23±28 days for TIA (p=0.029). Fifty-eight (29%) of the preceding symptomatic events occurred within seven days and 88 (44%) within 14 days before intervention.
ACUTE CASE RESULTS
Overall interventional success was achieved in 367 patients (97.9%). In the remaining cases, the stenosis could not be crossed or was not accessible. Placement of distal filter protection devices was possible in most patients (92%).
ADVERSE EVENTS
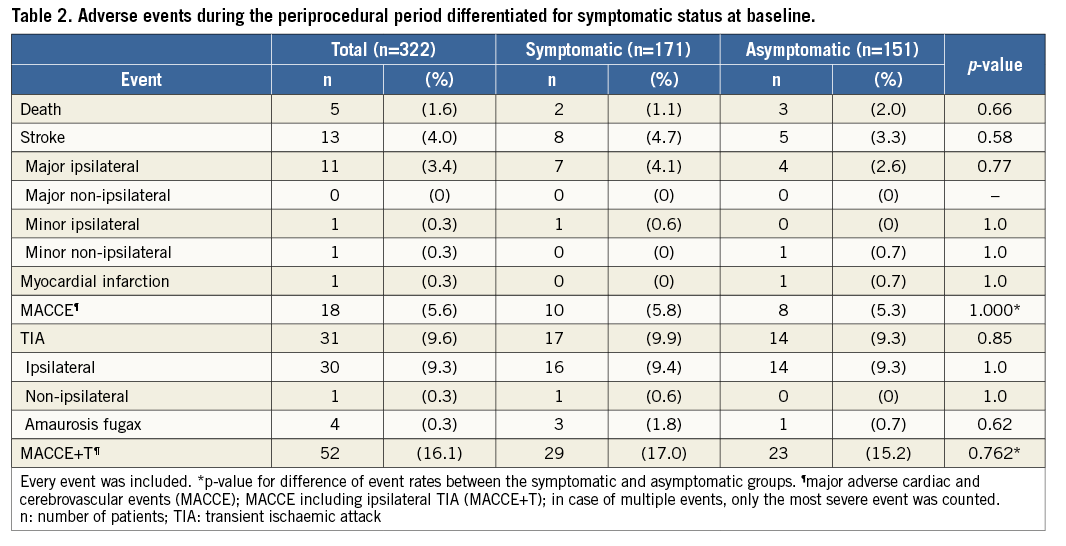
A total of 13 strokes (11 major ipsilateral, one minor ipsilateral, one minor contralateral), one MI and one death occurred during the periprocedural period (day zero to day 30 after CAS), resulting in a 30-day MACCE rate of 5.6% (n=18). Thirty-four patients experienced a transient periprocedural ipsilateral neurologic event, resulting in a periprocedural MACCE+T rate of 16.1%. Table 2 presents the 30-day adverse event rates, dichotomised by pre-procedural symptomatic versus asymptomatic status. An additional five patients (1.6%) experienced a MACCE+T between days 31 and 180. Eighty percent of TIAs occurred before hospital discharge (n=32, 8.5%). Fifty-three patients (14.1%) were lost to follow-up. At the final follow-up, the MACCE rate was 5.9% and the MACCE+T rate was 17.7%. This high combined adverse event rate was significantly related to the patients in subgroup C (n=77), categorised as very high risk before CAS according to the SAPPHIRE criteria, 23.4% of whom developed a MACCE+T event as compared to 11.4% in the groups with no obvious (A) or high risk (B) (A+B, n=298) together (OR: 2.6; 95% CI: 1.3-5.0; p=0.007). A significantly higher TIA rate (20.8%) within the subgroup at very high risk (C) accounted for this result as compared to 6.9% (subgroup A) and 7.4% (subgroup B).
RISK STRATIFICATION FOR PATIENT AND PROCEDURE-RELATED FACTORS
Overall, the periprocedural MACCE rate was not significantly different between symptomatic (5.8%) versus asymptomatic patients (5.3%) (p=1.0). There was no significant difference between the two groups for the MACCE+T rate (Table 2). Importantly, there appeared to be a differential impact on procedural risk among symptomatic patients depending on which type of cerebrovascular event had occurred previously. For patients who presented with a history of TIA, the risk for any peri-interventional adverse event increased significantly (OR for MACCE at 30 days: 2.77, p=0.039, and OR for MACCE+T at 30 days: 1.94, p=0.044) compared to all other patients. However, stroke alone was not a predictor of adverse events (Table 3, Table 4). Multiple logistic regression analysis used adjustment for factors that showed an association upon univariate analysis of MACCE (age, history of myocardial infarction and hyperlipidaemia at p<0.1) in addition to established covariates (sex, diabetes mellitus, previous CAS, coronary heart disease and clopidogrel intake). Adjustment did not change effect estimates of MACCE regarding the history of TIA (adjusted OR at 30 days: 2.88, p=0.039) (Table 3).
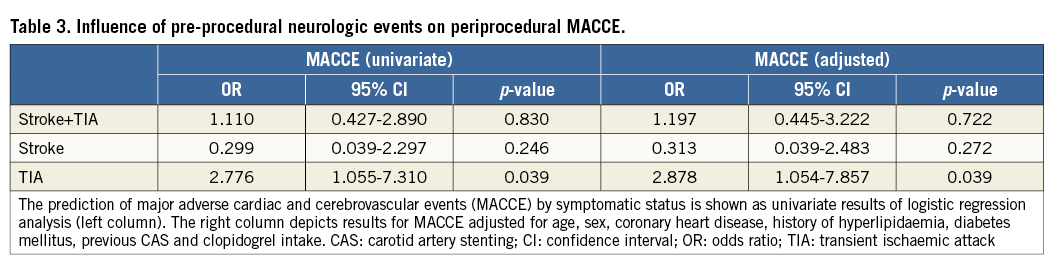
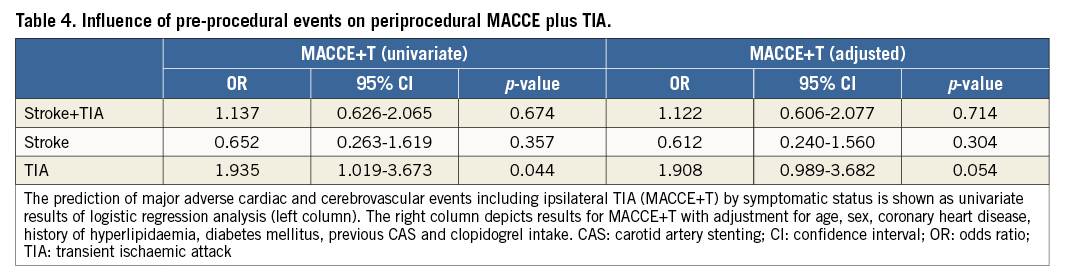
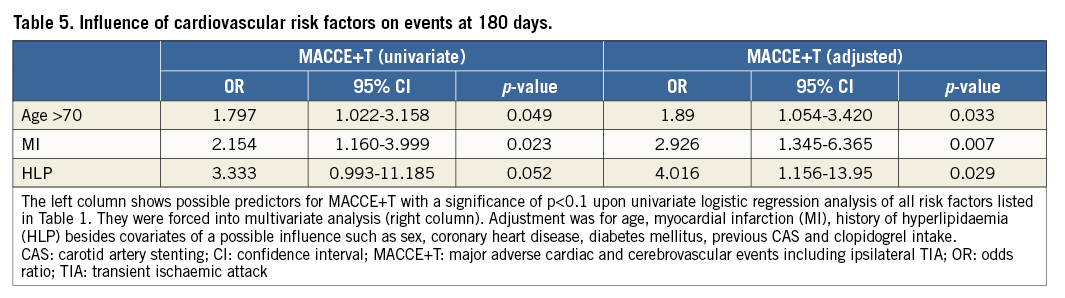
Prediction of MACCE+T at 180 days with cardiovascular risk factors used adjustment for factors as mentioned above for analysis of MACCE (Table 5). Age over 70 was significantly and independently associated with MACCE+T (adjusted OR: 1.89, p=0.033) (Table 5). In addition, a history of MI was an independent risk factor for MACCE+T (adjusted OR: 2.926, p=0.007) as well as a history of hyperlipidaemia (adjusted OR: 4.016, p=0.029), despite the fact that the vast majority (93%) were taking statin drugs (Table 5).
There was no difference between different stent types (open vs. closed cell) (MACCE +T at 30 days OR: 0.903; 95% CI: 0.492-1.659; p=0.761) and various filter protection devices (MACCE+T at 30 days OR: 0.647; 95% CI: 0.327-1.282; p=0.259).
Discussion
This prospective registry trial highlights the importance of TIA as a predictor of adverse outcomes following CAS. Specifically, we found that prior TIA was a significant risk factor for periprocedural MACCE and MACCE+T, while prior stroke was not. The discrepancy between stroke and TIA is most likely explained by the fact that there was a significantly greater delay to carotid revascularisation following a stroke, compared to those patients who presented with a TIA (mean difference=eight days, p=0.029 for difference). Importantly, the diagnosis of classic TIA is frequently associated with cerebral ischaemia irrespective of the duration of symptoms7. Several imaging trials have shown that approximately one third of TIA patients suffered from cerebral tissue injury, while being classified as non-stroke according to the classic 24-hour TIA definition7,13. While a history of classic TIA should be considered more seriously as a significant risk factor for procedural complications, this study does not provide any guidance on the optimal timing of revascularisation. There is high-level evidence that the risk of stroke recurrence is highest early on following a first stroke or TIA due to carotid stenosis, and it appears that early revascularisation is probably the best approach in general14.
We also noted a high combined MACCE+T event rate at 30 days (16.1%), due to a high incidence of TIA (9.6%) as compared to all MACCE (5.6%) (Table 2). The latter is approximately equal to the results of the randomised CREST trial (5.2%) which also included MI within the primary composite endpoint2. The 3.3% stroke and 2% death rates in asymptomatic patients are above the thresholds previously constituted5. The overall high event rate may, at least in part, be explained by a relevant number of patients at high and very high preinterventional risk according to the SAPPHIRE criteria12. In the randomised SAPPHIRE trial, a high-risk cohort was treated by CAS and compared to CEA. An even higher risk was given in 77 (20.5%) of our patients who fulfilled SAPPHIRE exclusion criteria. These patients were found to contribute to a significantly higher MACCE+T rate than the group at normal and high risk together (p=0.007). Furthermore, the TIA rate at discharge (8.5%) turned out to be relatively higher compared to the in-hospital rate of the Pro-CAS registry trial (6.0%)15. The high rate of periprocedural TIA could be partly due to the fact that a neurologist evaluated each patient before and after CAS, and transient focal neurological events were prospectively specified as an outcome of interest. In Pro-CAS, TIA rates varied between 8.2% with and 5.1% without a mandatory consultation of a neurologist before and after intervention, after hospital discharge15. Studies of patients undergoing magnetic resonance imaging following CAS have found radiographic infarct in half of patients, and thus it is highly likely that patients with transient neurologic symptoms after CAS have actually suffered a stroke, although the long-term implications remain uncertain16.
A striking result of our multiple regression analysis was that a history of MI was found to be an independent predictor for outcome after CAS at day 180 (MACCE+T: OR: 2.926, p=0.007). The influence of a history of coronary heart disease on outcome after CAS was described earlier by Hofmann et al17. However, the exact relationship between coronary heart disease and procedural complications has not been well characterised, and we did not find a report that analysed the association of MI as defined by contemporary standards. In our cohort, out of 75 patients with a history of MI, 20 became symptomatic during and after CAS: only one of these cases turned out to be another MI, the others being five strokes, four deaths and 10 TIAs. Thus, a history of MI does not merely predispose to recurrent cardiac events but displays an independent risk for both cardiac and cerebrovascular events after CAS. Therefore, prior MI should be one of the factors considered prior to undertaking revascularisation with CAS.
Hyperlipidaemia as a predictor of outcome after CAS has not been established in studies aiming at prospective risk stratification or in randomised trials comparing CAS with CEA. However, two registry trials suggested a potential value18,19. In our cohort, a history of hyperlipidaemia proved to be the strongest independent outcome predictor for MACCE+T after CAS at 180 days (OR: 4.016, p=0.029). Since 93% of all patients with known hyperlipidaemia were being treated with statins, this predictor may gain relevance for stratification of patients, even given lowered blood values. Hence it may be worth studying this predictor in depth within a larger controlled trial.
Age was shown to be another independent risk factor of importance for decision making concerning CAS and CEA. Many studies agree on this hypothesis but results are inconsistent with respect to the exact age cut-off point2-4,20. In a subgroup analysis of the CREST study, patients over 80 years of age showed a significantly increased risk for cardiovascular events following CAS2,21. In the SPACE study, age higher than 68 showed the most significant relative risk increase (2.7 vs. 10.8%, p=0.001)22. In the present analysis, age higher than 70 was an independent risk factor associated with a 1.9-fold increase of any cerebrovascular event after CAS (p=0.033), whereas age >80 was not. These results are consistent with a meta-analysis of randomised controlled trials that showed that increased age over 70 may lead to relatively better results in CEA23.
Limitations
This is not a randomised trial and, while registry studies may be limited by subjective patient selection, CAS patients in this study were selected in conformity with guidelines of the American Stroke Association and according to a careful assessment of symptomatic status by a neurologist. Follow-up for the 30-day and 180-day endpoints was completed in 86%, resulting in a possible selection bias. Finally, the number of procedures included in this analysis may have had limited power to detect other rare, clinically meaningful predictors of complications of CAS.
Conclusions
This study is among the first to highlight the clinical importance of TIA in the context of CAS, recording an increased number of TIA, relating worse outcome including TIA to high-risk patients and to the timing of CAS relative to the index event, and identifying a history of TIA as an underappreciated risk factor for the outcome after CAS besides a history of previous MI and hyperlipidaemia.
| Impact on daily practice There may be an increased awareness that patients with a previous TIA should be considered more seriously at risk for procedural complications in addition to patients with a history of MI, hyperlipidaemia and aged >70. Our findings suggest selection criteria should be studied further within very large multicentre trials, addressing the timing of CAS after TIA relative to stroke to improve outcomes. Peri-interventional TIA needs to be precisely monitored during and after CAS to ensure an improved internal and external validation of outcomes. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

