Abstract
BACKGROUND: Technological and surgical approaches to carotid artery stenting (CAS) have evolved. Modern randomised controlled trials comparing CAS and carotid endarterectomy (CEA) are limited, and information about updated post-intervention outcomes are mostly from retrospective, small studies.
AIMS: This study aims to compare the 30-day outcomes of stroke, transient ischaemic attack (TIA), acute myocardial infarction (AMI) and death with propensity-matched groups of CEA and CAS in asymptomatic and symptomatic patients over a recent study period of new CAS technologies and approaches.
METHODS: A retrospective, observational, multicentre analysis was conducted including consecutive symptomatic and asymptomatic patients treated with either primary CEA or CAS for internal carotid artery stenosis, between 2015 and 2022. Patients were propensity score-matched based on comorbidities and assessed according to symptom status. Primary endpoints include composite ipsilateral stroke, TIA, AMI and death within 30 days. Secondary endpoints include technical success and length of hospital stay.
RESULTS: From a cohort of 1,110 patients, propensity matching produced 269 distinct treatment pairs (n=538). Most patients were asymptomatic (n=456, 85%). All 6 strokes were minor (CEA=2; CAS=4) and registered among asymptomatic patients. One AMI (CEA) and 1 patient death (CAS) were reported among symptomatic patients. Composite stroke/AMI/death were not significantly different between both types of symptom status and both revascularisation techniques (p=0.44 and p=1, respectively). Technical success was 100%. The length of hospital stay was significantly shorter in asymptomatic patients treated with CAS compared to those treated with CEA (p=0.05), but no difference was registered among symptomatic patients (p=0.32).
CONCLUSIONS: Propensity-matched analysis suggests that CAS has similar postprocedural outcomes for stroke, AMI and death at 30 days compared to CEA.
Extracranial carotid artery disease accounts for 15-20% of all ischaemic strokes1. Carotid revascularisation has played a key role in primary and secondary prevention of cerebrovascular events since previous landmark randomised controlled trials (RCTs) contributed to the currently accepted indications for intervention234. Carotid revascularisation is performed by either traditional carotid endarterectomy (CEA) or carotid artery stenting (CAS). CEA is commonly adopted for patients at low risk of stroke/death, and CAS is indicated for selected patients at high risk of neurological and cardiovascular complications56.
CAS has been found to compare favourably with CEA for postprocedural prevention of primary and secondary stroke in patients with significant extracranial internal carotid artery (ICA) stenosis, for both symptomatic and asymptomatic patients4789. However, CAS has faced significant barriers to adoption due to its higher rates of procedural stroke related to procedural complications, primarily involving embolic events. In recent years, these complication rates have decreased because of technical advances, improved operator experience, better interventional strategies and advanced stent design1011.
The authors hypothesise that updated data may reflect advances in the CAS procedure compared to CEA. Real-world data (RWD) can contribute to updated assessments of carotid revascularisation techniques if the innate problems associated with retrospective study design can be accounted for. Propensity-matched analysis can remove selection bias, by creating homogeneous groups, similar to those created in prospectively designed RCTs. Furthermore, as there are fortunately few major events following modern carotid revascularisation, composite outcomes can produce more meaningful data for analysis in small patient populations.
This retrospective, observational, multicentre study aims to compare the 30-day composite outcome of stroke, myocardial infarction and death, and transient ischaemic attack (TIA) with propensity-matched groups including asymptomatic and symptomatic patients treated with CEA and CAS for carotid stenosis over a recent study period that has incorporated new CAS technologies and approaches.
Methods
STUDY DESIGN AND PATIENTS
This multicentre, retrospective study enrolled consecutive patients with significant internal carotid artery (ICA) stenosis who had undergone either primary CEA or CAS for carotid revascularisation treatment in 1 of 2 high-volume centres between January 2015 and July 2022. One centre included cardiology interventionalists who performed CAS only (around 80-100 CAS procedures per year); patients referred to their centre had previously been screened for CAS inclusion. The other centre included vascular surgeons who performed CEA only (around 100-120 CEA procedures per year). Patients provided written informed consent for the procedure, and the study protocol was approved by the referral ethics committees of the 2 participating centres (CE 923/2021/OSS/AOUPR, AVEN and CE 9953/2019 I.5/93, CEROM). This study was conducted in accordance with the Declaration of Helsinki.
Patients were selected for treatment according to standard guidelines: patients who were symptomatic (carotid stenosis ≥50% that led to a carotid territory symptom[s] within the preceding 6 months) or asymptomatic (carotid stenosis ≥70% without symptoms within the preceding 6 months) and considered suitable for revascularisation treatment6. The patient exclusion criteria specified complete ipsilateral carotid occlusion or near-occlusion, restenosis after CEA or CAS, myocardial infarction within 72 hours, intracranial haemorrhage in the preceding 12 months or emergent neurological patients (modified Rankin Scale >3/area of infarction >one third of the middle cerebral artery territory/altered consciousness). Study criteria further specified the exclusion of patients with incomplete clinical data and those with less than 30-day follow-up.
A full patient cohort database was assembled from the 2 participating centres, from which a propensity-matched analysis, based on confounding baseline demographic and comorbid factors, was performed to create matched pairs for assessment. For the 30-day outcome analysis, patients were also grouped according to their baseline symptom presentation.
DATA COLLECTION AND DEFINITIONS
Data were retrospectively collected in a dedicated database including demographic and comorbid variables: hypertension, diabetes mellitus (DM), coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), atrial fibrillation, smoking status, dyslipidaemia, chronic kidney disease (CKD; defined by an estimated glomerular filtration rate <60 ml/h) and on haemodialysis treatment (CKD-HD). Additionally, lesion/stenosis characteristics and symptom status were recorded, as well as operative, complications and outcome data.
All patients were preoperatively evaluated by Doppler ultrasound (DUS), computed tomography (CT) angiography (CTA) of supra-aortic vessels and cerebral CT without contrast medium. Percentage stenosis was assessed by CTA according to North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria2.
Lesion characteristics were preoperatively evaluated by DUS performed by vascular surgeons or interventional cardiologists, followed by CTA of supra-aortic vessels. Intraoperative digital subtraction angiography (DSA) was performed only in the case of an endovascular treatment. Lesion types were classified on CTA as ulcered plaque, defined as contrast medium extending beyond the vascular lumen (≥1 mm in at least 2 scanning planes), or severe calcification, defined as radiopacities (≥50% of the arterial lumen diameter) on both sides. Technical success was defined as effective stent placement/revascularisation with residual stenosis <30%, assessed by postoperative DUS.
TIA was defined as a brief episode of neurological dysfunction resulting from focal, temporary cerebral ischaemia that was not associated with acute cerebral infarction and persisted <24 hours612. Ischaemic stroke was defined by an ipsilateral neurological dysfunction persisting ≥24 hours or until death6. All strokes were categorised as minor (National Institute of Health Stroke Scale [NIHSS] ≤5) or major (NIHSS >5)13.
Acute myocardial infarction (AMI) was defined by the detection of a rise and/or fall of cardiac biomarker values, preferably cardiac troponin, with at least 1 value above the 99th percentile upper reference limit and with at least one of the following:
• Symptoms of ischaemia
• New or presumed new significant ST-segment T-wave changes or new left bundle branch block
• Development of pathological Q waves in the electrocardiogram
• Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
• Identification of an intracoronary thrombus by angiography or autopsy14.
Death for any reason was registered according to the date of the event reported by the patient’s family or the institution’s database.
CAROTID REVASCULARISATION TREATMENT TECHNIQUES
All CEA were performed under general anaesthesia with conscious sedation. Briefly, after surgical incision, during common carotid dissection and before cross-clamping, remifentanil infusion is slowly reduced until patients are conscious. The patient is instructed to squeeze a soft toy with the contralateral hand during common carotid artery (CCA) cross-clamping (for 2 consecutive minutes) and carotid bifurcation clamping (for 1 minute). If this neurological tolerance test (NTT) indicates neurological symptoms consistent with cerebral hypoperfusion or ischaemia, a remifentanil infusion is implemented, and a shunt is positioned. At procedure completion, the remifentanil infusion is reduced again for a final NTT151617. Patch angioplasty and eversion were the only surgical techniques used. No intraÂoperative quality control was performed during CEA.
All CAS procedures were performed by femoral or radial percutaneous access. During most procedures, either a proximal (Mo.Ma [Medtronic]) or distal (SpiderFX [Medtronic] or FilterWire EZ [Boston Scientific]) embolic protection device (EPD) was used; the device was selected according to the individual patient’s anatomical characteristics, including arch anatomy, external carotid artery occlusion, and the expected technical difficulty of crossing ICA lesions. For CAS, second- or first- generation stents were used, including Roadsaver (Terumo) and CGuard (InspireMD), or Carotid WALLSTENT (Boston Scientific), XACT (Abbott) and Cristallo Ideale (Medtronic), respectively.
MEDICAL THERAPY
Patients treated with CEA received single antiplatelet therapy (SAPT) at a standard dose at least 1 week before surgery, and this was maintained indefinitely post-procedure. In patients already under oral anticoagulant (OAC) therapy, preoperative bridging with low-molecular-weight heparin was required only for patients on oral vitamin K antagonists. Intraprocedural administration of unfractionated heparin (5,000 IU) was administered at the time of cross-clamping. Protamine was administered in selected cases to improve bleeding control. Postoperative bridging with low-molecular-weight heparin was indicated with SAPT, recommended for 1 month, with OAC resumed only after surgical suture removal.
For CAS procedures, all patients received dual antiplatelet therapy (DAPT) at a standard dose >2 days before the procedure. In cases of urgent interventions, intraprocedural clopidogrel loading was performed. Intraprocedural administration of unfractionated heparin (70-100 IU/kg) was administered at the time of the percutaneous puncture to maintain an activated clotting time (ACT) >250 seconds. In case of ACT prolongation beyond 250 seconds, protamine was administered after the procedure. To prevent baroreceptor-stimulated bradycardia or hypotension, a single intraprocedural atropine bolus dose of 1 mg was administered before post-dilation. Following the procedure, clopidogrel therapy was continued for 3 months, and SAPT was continued indefinitely. If patients were taking an OAC preoperatively, either a SAPT therapy or a combined DAPT and OAC therapy were recommended, according to the patients’ major bleeding risk assessment.
FOLLOW-UP
Follow-up protocol specified evaluation at 30 days by clinical, carotid DUS and neurological examinations.
ENDPOINTS
The study’s primary endpoint was the 30-day risk of ipsilateral stroke, TIA, AMI and death. Secondary outcomes included technical success and hospital stay duration.
PROPENSITY SCORE-MATCHING ANALYSIS
Propensity score matching (PSM) was performed to account for differences in the estimation of average treatment effects of CEA and CAS introduced by a retrospective, observational study, thus giving the probability of being in a treatment group, given the set of observed covariates. For PSM analysis Stata/SE 17.0 software (StataCorp) was used on a 1:1 ratio based on a predefined calliper width without replacement. The PSM analysis used the nearest-number matching method with an optimal calliper width of 0.1 to minimise the mean squared error of the estimated treatment effect. Covariates (confounding factors) included hypertension, CAD, atrial fibrillation, dyslipidaemia, CKD, symptomatic lesions, % carotid stenosis, ulcered plaque and severe calcification.
STATISTICAL ANALYSIS
Data were recorded, and calculation of the preoperative percentage of lumen reduction was performed with Microsoft Excel (Microsoft). Qualitative data were reported as absolute number and percentage, quantitative data as mean±standard deviation (SD). The chi-square test was used to evaluate the distribution of preoperative risk factors and lesion characteristics data between the CEA and CAS groups; Fisher’s exact test was adopted in cases of values <5. All the endpoints were evaluated with the chi-square test; Fisher’s exact test was adopted in cases of values <5. Statistical analyses were performed with SPSS software (version 13.0; IBM) and Stata/SE 17.0 software (StataCorp). A p-value<0.05 was considered statistically significant.
Results
ORIGINAL COHORT BASELINE CHARACTERISTICS
A total of 1,110 treated ICA stenoses met the inclusion criteria. Of these, 610 (55%) had undergone CEA (67% male, mean age 73.4±7.8 years) in the first high-volume centre and 500 (45%) had undergone CAS (66% male, mean age 73.5±9.0 years) in the second high-volume centre, over the 7-year study period. No emergent, neurologically unstable patients were treated at these centres during the same period. Technical success was achieved for all patients.
According to the carotid treatment received (CEA or CAS), comorbidity and lesion characteristics were mostly heteroÂgeneous (Table 1). Patients who underwent CAS tended to have significantly more cardiovascular risk factors, including CAD (p<0.001), hypertension (p=0.02), dyslipidaemia (p<0.001), atrial fibrillation (p<0.001), and CKD (p<0.001), and were significantly less likely to be symptomatic at presentation (p<0.001) with less calcified plaques (p<0.01), but had significantly higher degrees of stenosis (p<0.001) and ulcered plaques (p<0.001).
Table 1. Baseline demographic, comorbid and lesion characteristics of original and propensity-matched cohorts.
| Original cohort, n=1,100 | Propensity-matched cohort, n=538 (49) | |||||||
|---|---|---|---|---|---|---|---|---|
| All patients n=1,100 | CEA n=610 (55) | CAS n=500 (45) | p-value | All PM cohort n=538 | CEA n=269 (50) | CAS n=269 (50) | p-value | |
| Demographic variables | ||||||||
| Age, years | 73.4±8.3 | 73.4±7.8 | 73.5±9.0 | 0.83 | 72.8±8.1 | 73.6±7.3 | 72.1±8.9 | 0.34 |
| Sex, male | 738 (66.5) | 406 (66.6) | 332 (66.4) | 0.96 | 364 (67.7) | 187 (69.5) | 177 (65.8) | 0.36 |
| Comorbidities | ||||||||
| Hypertension | 976 (87.9) | 524 (85.9) | 452 (90.4) | 0.02 | 464 (86.2) | 233 (86.6) | 231 (85.9) | 0.80 |
| DM | 338 (30.5) | 198 (32.5) | 140 (28.0) | 0.11 | 171 (31.8) | 85 (31.6) | 86 (32.0) | 1 |
| CAD | 320 (28.8) | 137 (22.5) | 183 (36.6) | <0.001 | 160 (29.7) | 83 (30.9) | 77 (28.6) | 0.57 |
| COPD | 168 (15.1) | 91 (14.9) | 77 (15.4) | 0.82 | 89 (16.5) | 41 (15.2) | 48 (17.8) | 0.42 |
| Atrial fibrillation | 89 (8.0) | 31 (5.1) | 58 (11.6) | <0.001 | 45 (8.4) | 21 (7.8) | 24 (8.9) | 0.64 |
| Smoker, current | 238 (21.4) | 138 (22.6) | 100 (20.0) | 0.28 | 119 (22.1) | 57 (21.1) | 62 (23.0) | 0.60 |
| Smoker, former | 410 (36.9) | 214 (35.1) | 196 (39.2) | 0.16 | 208 (38.7) | 110 (40.9) | 98 (36.4) | 0.29 |
| Dyslipidaemia | 903 (81.4) | 454 (74.4) | 449 (89.8) | <0.001 | 458 (85.1) | 229 (85.1) | 229 (85.1) | 1 |
| CKD | 277 (25.0) | 78 (12.8) | 199 (39.8) | <0.001 | 125 (23.2) | 65 (24.2) | 60 (22.3) | 0.61 |
| CKD-HD | 7 (0.6) | 5 (0.8) | 2 (0.4) | 0.32 | 4 (0.7) | 2 (0.7) | 2 (0.7) | 1 |
| Lesion characteristics | ||||||||
| Symptomatic | 197 (17.7) | 137 (22.5) | 60 (12.0) | <0.001 | 82 (15.2) | 37 (13.8) | 45 (16.7) | 0.34 |
| Carotid stenosis, % | 80.1±9.5 | 76.7±9.5 | 84.1±7.6 | <0.001 | 81.5±8 | 81.5±8.4 | 81.5±7.6 | 0.93 |
| Lesion side, right | 581 (52.3) | 328 (53.8) | 253 (50.6) | 0.37 | 295 (54.8) | 150 (55.8) | 145 (53.9) | 0.67 |
| Ulcered plaque | 95 (8.6) | 33 (5.4) | 62 (12.4) | <0.001 | 49 (9.1) | 23 (8.6) | 26 (9.7) | 0.65 |
| Severe calcification | 310 (27.9) | 225 (36.9) | 85 (17.0) | <0.001 | 121 (22.5) | 52 (19.3) | 69 (25.7) | 0.08 |
| Data are presented as mean±SD or n (%). p-values in bold indicate statistical significance. CAD: coronary artery disease; CAS: carotid artery stenting; CEA: carotid endarterectomy; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; HD: haemodialysis; PM: propensity-matched; SD: standard deviation | ||||||||
PROPENSITY-MATCHED COHORT
A 1:1 propensity match generated 269 distinct treatment pairs (538 patients) (Table 1). PSM included all comorbidities that were significantly different among the whole population (hypertension, CAD, atrial fibrillation, dyslipidaemia and CKD), symptom status and lesion characteristics. PSM ensured a consistent sample size (50% CEA, 50% CAS) of a more homogeneous population (Figure 1). Most patients were asymptomatic (n=456, 84.8%).
CEA treatment was characterised by patch angioplasty for most procedures (55%). Most CAS procedures were achieved via femoral percutaneous access (88.5%), and an EPD was employed in all cases (100%) (Mo.Ma was the most used device: 64.6%). Second-generation stents were deployed in almost half of CAS procedures (47.6%) (Table 2).
Table 1. Baseline demographic, comorbid and lesion characteristics of original and propensity-matched cohorts.
| Original cohort, n=1,100 | Propensity-matched cohort, n=538 (49) | |||||||
|---|---|---|---|---|---|---|---|---|
| All patients n=1,100 | CEA n=610 (55) | CAS n=500 (45) | p-value | All PM cohort n=538 | CEA n=269 (50) | CAS n=269 (50) | p-value | |
| Demographic variables | ||||||||
| Age, years | 73.4±8.3 | 73.4±7.8 | 73.5±9.0 | 0.83 | 72.8±8.1 | 73.6±7.3 | 72.1±8.9 | 0.34 |
| Sex, male | 738 (66.5) | 406 (66.6) | 332 (66.4) | 0.96 | 364 (67.7) | 187 (69.5) | 177 (65.8) | 0.36 |
| Comorbidities | ||||||||
| Hypertension | 976 (87.9) | 524 (85.9) | 452 (90.4) | 0.02 | 464 (86.2) | 233 (86.6) | 231 (85.9) | 0.80 |
| DM | 338 (30.5) | 198 (32.5) | 140 (28.0) | 0.11 | 171 (31.8) | 85 (31.6) | 86 (32.0) | 1 |
| CAD | 320 (28.8) | 137 (22.5) | 183 (36.6) | <0.001 | 160 (29.7) | 83 (30.9) | 77 (28.6) | 0.57 |
| COPD | 168 (15.1) | 91 (14.9) | 77 (15.4) | 0.82 | 89 (16.5) | 41 (15.2) | 48 (17.8) | 0.42 |
| Atrial fibrillation | 89 (8.0) | 31 (5.1) | 58 (11.6) | <0.001 | 45 (8.4) | 21 (7.8) | 24 (8.9) | 0.64 |
| Smoker, current | 238 (21.4) | 138 (22.6) | 100 (20.0) | 0.28 | 119 (22.1) | 57 (21.1) | 62 (23.0) | 0.60 |
| Smoker, former | 410 (36.9) | 214 (35.1) | 196 (39.2) | 0.16 | 208 (38.7) | 110 (40.9) | 98 (36.4) | 0.29 |
| Dyslipidaemia | 903 (81.4) | 454 (74.4) | 449 (89.8) | <0.001 | 458 (85.1) | 229 (85.1) | 229 (85.1) | 1 |
| CKD | 277 (25.0) | 78 (12.8) | 199 (39.8) | <0.001 | 125 (23.2) | 65 (24.2) | 60 (22.3) | 0.61 |
| CKD-HD | 7 (0.6) | 5 (0.8) | 2 (0.4) | 0.32 | 4 (0.7) | 2 (0.7) | 2 (0.7) | 1 |
| Lesion characteristics | ||||||||
| Symptomatic | 197 (17.7) | 137 (22.5) | 60 (12.0) | <0.001 | 82 (15.2) | 37 (13.8) | 45 (16.7) | 0.34 |
| Carotid stenosis, % | 80.1±9.5 | 76.7±9.5 | 84.1±7.6 | <0.001 | 81.5±8 | 81.5±8.4 | 81.5±7.6 | 0.93 |
| Lesion side, right | 581 (52.3) | 328 (53.8) | 253 (50.6) | 0.37 | 295 (54.8) | 150 (55.8) | 145 (53.9) | 0.67 |
| Ulcered plaque | 95 (8.6) | 33 (5.4) | 62 (12.4) | <0.001 | 49 (9.1) | 23 (8.6) | 26 (9.7) | 0.65 |
| Severe calcification | 310 (27.9) | 225 (36.9) | 85 (17.0) | <0.001 | 121 (22.5) | 52 (19.3) | 69 (25.7) | 0.08 |
| Data are presented as mean±SD or n (%). p-values in bold indicate statistical significance. CAD: coronary artery disease; CAS: carotid artery stenting; CEA: carotid endarterectomy; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; HD: haemodialysis; PM: propensity-matched; SD: standard deviation | ||||||||
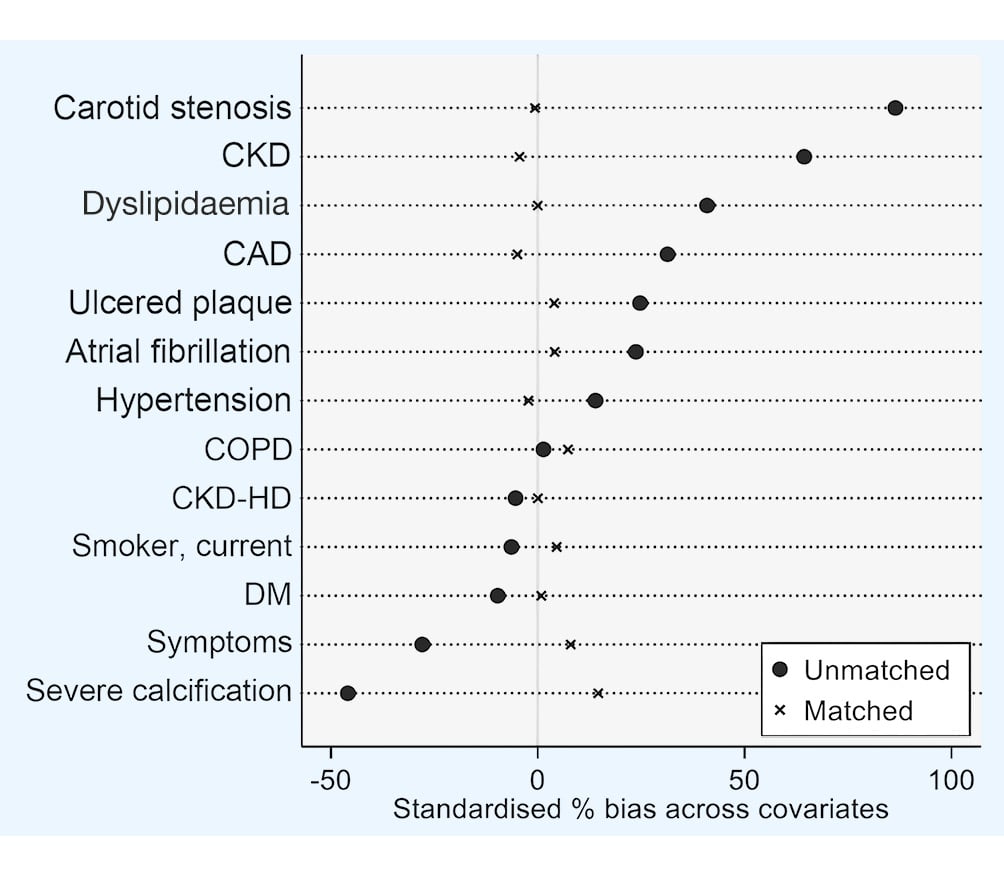
Figure 1. Propensity score-matched groups of patients, based on selected preoperative variables. CAD: coronary artery disease; CKD: chronic kidney disease; CKD-HD: chronic kidney disease on haemodialysis; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus
Table 2. Operative data for 269 propensity score-matched patients treated with carotid artery stenting, according to symptomatic status at presentation: asymptomatic or symptomatic.
| Asymptomatic n=224 (83.3) | Symptomatic n=45 (16.7) | p-value* | |
|---|---|---|---|
| CAS intervention (n=269) | |||
| Predilation | 25 (11.2) | 3 (6.7) | 0.59 |
| Post-dilation | 216 (96.4) | 44 (97.8) | 1 |
| Percutaneous access site | |||
| Femoral | 196 (87.5) | 42 (93.3) | 0.32 |
| Radial | 28 (12.5) | 3 (6.7) | |
| Cerebral protection | |||
| Proximal | 142 (63.4) | 32 (71.1) | 0.37 |
| Distal | 82 (36.6) | 13 (28.9) | |
| Contrast medium, ml | 170.3±55.9 | 171.3±40.8 | 0.92 |
| Stents deployed | 107 (47.8) | 21 (46.7) | 0.89 |
| Single stent (n=1) | 220 (98.2) | 45 (100) | 1 |
| Multiple stents (n≥2) | 4 (1.8) | 0 (0) | |
| Stent length, mm | 31.6±9.2 | 31.3±8.4 | 0.86 |
| Second-generation stents | |||
| Roadsaver | 91 (40.6) | 19 (42.2) | 0.70 |
| CGuard | 12 (5.4) | 2 (4.4) | |
| First-generation stents | |||
| Carotid WALLSTENT | 27 (12.1) | 8 (17.8) | 0.70 |
| XACT | 80 (35.7) | 15 (33.3) | |
| Cristallo Ideale | 14 (6.3) | 1 (2.2) | |
| Debris observed in filter of the protection system (with naked eye) | 7 (3.1) | 1 (2.2) | 1 |
| Data are presented as mean±SD or n (%). *The chi-square test was used to evaluate qualitative distribution between the 2 groups (Fisher’s exact test if cases <5). Means were compared with the t-test. CAS: carotid artery stenting | |||
ENDPOINTS
All 6 stroke events were minor and registered among asymptomatic patients; 2 had been treated with CEA and 4 with CAS (p=0.44). Three of the 4 CAS interventions deployed a distal filter for cerebral protection. All 4 of these patients had been treated with post-dilation, and the stents used included XACT (n=1), Cristallo Ideale (n=1), Roadsaver (n=1) and Carotid WALLSTENT (n=1). Only 1 patient had been treated with a second-generation stent (Roadsaver).
One AMI was registered in a symptomatic patient treated with CEA. One patient death was registered in a symptomatic patient who had been treated with CAS (p=0.5): a 93-year-old hypertensive, male patient had been preoperatively regisÂtered with dyslipidaemia and atrial fibrillation on OAC. Composite stroke/AMI/death rates were not significantly different between both types of symptom status and both revascularisation techniques (p=0.44 and p=1, respectively).
Technical success was 100%. The hospital stay was significantly shorter for asymptomatic patients treated with CAS than for those treated with CEA (p=0.05), but no difference was registered among symptomatic patients (p=0.32) (Table 3).
Table 3. Thirty-day outcomes associated with carotid endarterectomy (CEA) and carotid artery stenting (CAS) after propensity score matching.
| Outcomes | All patients | Asymptomatic n=456 (84.8%) | Symptomatic n=82 (15.2%) | ||||
|---|---|---|---|---|---|---|---|
| n=538 (100) | CEA n=232 (50.9) | CAS n=224 (49.1) | p-value | CEA n=37 (45.1) | CAS n=45 (54.9) | p-value | |
| Stroke - minor | 6 (1.1) | 2 (0.9) | 4 (1.8) | 0.44 | - | - | - |
| Stroke - major | - | - | - | - | - | - | - |
| TIA | 6 (1.1) | 2 (0.9) | 3 (1.3) | 0.68 | - | 1 (2.2) | 1 |
| AMI | 1 (0.2) | - | - | - | 1 (2.7) | - | 0.45 |
| Death | 1 (0.2) | - | - | - | - | 1 (2.2) | 1 |
| Stroke+death | 7 (1.3) | 2 (0.9) | 4 (1.8) | 0.44 | - | 1 (2.2) | 1 |
| Stroke+AMI+death | 8 (1.5) | 2 (0.9) | 4 (1.8) | 0.44 | 1 (2.7) | 1 (2.2) | 1 |
| Hospitalisation, days | 3.6±2.7 | 3.9±2.1 | 3.2±3.0 | 0.005 | 4.1±2.9 | 3.5±3.3 | 0.32 |
| Data are presented as n (%) or mean±SD. p-values in bold indicate statistical significance. AMI: acute myocardial infarction; CAS: carotid artery stenting; CEA: carotid endarterectomy; SD: standard deviation; TIA: transient ischaemic attack | |||||||
Discussion
This propensity-matched cohort of patients treated with CAS or CEA for asymptomatic or symptomatic carotid stenosis suggests there is no significant difference in the choice of CEA or CAS for carotid revascularisation in terms of death/stroke/AMI within 30 days of the procedure.
Large-scale RCTs have established the superiority of CEA over medical management in patients with symptomatic and asymptomatic carotid artery disease181920. CAS has been associated with an increase in short-term morbidity and mortality compared to CEA621, but RCTs comparing CAS and CEA have largely reported similar long-term protective effects against fatal and disabling stroke6822. However, recent improvements in medical treatments have reduced absolute stroke rates following CEA and CAS. Furthermore, second-generation stent designs and updated procedural approaches have made CAS safer in terms of stroke risk2324, but current guidelines reserve CAS for selected patients who are classified at high surgical risk5.
Among the original cohort, more comorbidities were regisÂtered in patients treated with CAS, with significantly more patients with coronary artery-related illness, dyslipidaemia and CKD compared to those treated with CEA. This finding is not unexpected, as preoperative variables have a significant influence on the indication for the type of revascularisation. Therefore, a direct comparison between the RWD on CAS and CEA cannot be made.
RCTs are considered the highest level of evidence in clinical research due to their specific protocol design, random assignment of participants and inclusion of a control group. However, they can be both cumbersome and expensive to conduct, often delaying timely results. RWD can provide updated information, including modern techniques and devices, but are plagued by issues of selection bias, confounding factors, unmeasured variables and the lack of a control group. Furthermore, the quality, accuracy and completeness of RWD can create bias and affect the validity and generalisability of the findings. Propensity score analysis (PSA) is a statistical method used to address issues of confounding and selection bias in RWD by balancing the distribution of observed covariates between treatment groups through the creation of a pseudorandomisation. A previous PSA study comparing CEA and CAS, including first-generation stents, has already been published25.
There is a need for updated comparative, immediate outcomes for CAS and CEA in the literature, because recent second-generation stents with micromesh technology theoretically offer superior protection against ischaemic complications, with improved prevention for plaque prolapse and subsequent debris migration26. A meta-analysis comparing first- and second-generation stents found a significant reduction in 30-day peri- and postprocedural strokes, despite considerable heteroÂgeneity among studies and outcomes23. Large, randomised trials (the Carotid Revascularization Endarterectomy versus Stenting Trial [CREST] and Carotid Angioplasty and Stenting Versus Endarterectomy in Asymptomatic Subjects Who Are at Standard Risk for Carotid Endarterectomy With Significant Extracranial Carotid Stenotic Disease [ACT I]) comparing standard-risk patients treated with CAS and CEA reported comparable composite 30-day primary outcomes, but with higher minor stroke rates in the CAS cohort427. Our study reports an overall 30-day composite stroke/AMI/death rate of 1%, with only 1 stroke registered in a patient treated with a second-Âgeneration stent, which is in line with modern CAS series reporting second-generation stent outcomes in a few selected patients, with a stroke occurrence ranging from 0-1.25%2628293031.
A protection device was employed for all CAS procedures, with over 2/3 of patients protected with proximal protection and 1/3 with distal protection. However, 75% of stroke events occurred in patients that had been treated with distal embolic protection and a first-generation stent. The small number of events did not allow for a specific statistical analysis of these findings, as has been reported in many other studies comparing use of an EPD in stenting with no use2426.
The current study applied the definition of asymptomatic status based on the absence of events over the previous 6 months. The most recent Italian guidelines suggest that this definition should be updated to 3 months5. These more recent guidelines were not able to be followed in this study, but it would surely have reduced the representation of symptomatic patients in this cohort. However, it would not have influenced the outcome, as few events were registered among the symptomatic patients in this series.
Limitations
Several limitations of this multicentre, retrospective study should be recognised. This study was designed as a retrospective observational study and non-random differences between the CEA and CAS groups were registered. PSA was used to reduce possible selection bias and confounder effects, by creating 2 balanced groups. However, PSA cannot account for unmeasured variables, and patients treated with CAS are at higher risk for surgery (according to the guidelines’ patient selection criteria) and, therefore, at higher risk of perioperative stroke. Clinical data (other than the study endpoints) and selection data were incomplete, and this study’s clinical success and number of excluded patients could not be reported. Percentage of stenosis was evaluated preoperatively with CTA in all patients, and these measurements may have overestimated stenosis32. Due to the low rate of events both prior to and after PSA matching, the statistical strength of our outcomes is restricted, and data should be interpreted with caution. While PSA cannot eliminate all the risk of unmeasured confounding factors, PSA provides a more rigorous approach to estimating treatment effects than traditional observational analyses. In our matching, preoperative antiplatelet and anticoagulation medications, plaque morphologies and time intervals between the event and revascularisation treatment among symptomatic patients were not included, despite being important determinants in CAS and CEA outcomes.
Conclusions
A PSA-matched cohort of CEA and CAS patients provides more reliable and robust evidence from real-world data, enabling informed decision-making in clinical practice and healthcare policy. Our study reports a similar 30-day composite stroke/AMI/death rate in asymptomatic and symptomatic patients matched for comorbidities.
Impact on daily practice
Previous large-scale randomised trials established the superiority of carotid endarterectomy (CEA) over medical management in patients with carotid artery disease. Guidelines reserve carotid artery stenting (CAS) for selected patients at high surgical risk. Therefore, patients treated with CEA or CAS are different according to their baseline risk (clinical and anatomical variables), limiting any retrospective comparison of the two treatment techniques.
Propensity score-matching analysis accounts for differences in treatment populations, creating a pseudorandomisation. Matched pairs can then be compared, in a similar way to randomised groups in prospective, controlled trials.
This propensity-matched analysis of CEA versus CAS for the treatment of extracranial carotid artery stenosis, based on differences in baseline comorbid features and lesion characteristics, suggests that CAS is not inferior to CEA in terms of 30-day composite stroke/acute myocardial infarction/death rates in asymptomatic and symptomatic patients.
Acknowledgements
The authors would like to thank Maurizio Rossi from the Department of Clinical and Experimental Medicine, University Hospital of Parma (Parma, Italy) for his statistical analysis assistance.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors received no financial support for the research, authorship, or publication of this article.
Conflict of interest statement
R. Nerla has received consulting fees from Medtronic and Terumo. F. Castriota has received consulting and proctoring fees from Abbott, Boston Scientific, Medtronic, and Terumo. The other authors have no conflicts of interest to declare.

