Abstract
Aims: This prospective registry was designed to evaluate the early and long-term incidence of clinical events in patients with carotid obstructive disease (COD), after carotid artery revascularisation selected by consensus of a cardiovascular team.
Methods and results: 403 consecutive patients with COD scheduled for carotid revascularisation were included: 130 were treated with carotid endarterectomy (CEA) and 273 with carotid artery stenting (CAS). Propensity score matching was performed to assemble a cohort of patients in whom all baseline covariates would be well balanced. The occurrence of major adverse cardiac and cerebrovascular events (MACCE), including any death, non-fatal myocardial infarction or stroke, was assessed at 30 days and at long-term follow-up. The incidence of MACCE at 30 days was 4.0% (95% confidence interval: 2.1 to 6.0), without any significant difference between the CAS and CEA groups in unmatched and matched populations. The cumulative freedom from MACCE at two-year follow-up was 80.5%±0.94%, with no statistically significant differences between the CAS and CEA groups, both in the total population and in the matched cohort.
Conclusions: In this registry of patients undergoing carotid artery revascularisation selected by consensus of a cardiovascular team, the early and long-term incidence of clinical events is up to standard.
Introduction
Carotid artery stenting (CAS) and carotid endarterectomy (CEA), when performed by highly qualified operators, are both safe and effective options for treating carotid artery stenosis, an important cause of ischaemic stroke. Most of the randomised clinical trials comparing CAS and CEA have presented several methodological limitations and provided controversial results1,2. Therefore, optimal treatment selection for each given patient is the eventual method by which the most favourable outcomes are achieved. Indeed, recent guidelines3,4 and consensus documents5 recommend an individual, tailor-made approach to the patient with carotid obstructive disease (COD).
This tailored strategy has been employed in our institution where both interventional cardiologists and vascular surgeons are experienced and jointly evaluate each single patient with COD, in order to identify the most appropriate approach, based on current scientific evidence and good clinical judgement. We sought to evaluate the early and long-term incidence of clinical events in an all-comers population of patients with COD, after carotid artery revascularisation selected by consensus of a cardiovascular team.
Methods
PATIENT POPULATION AND SELECTION CRITERIA
In this prospective, single-centre registry, we included all consecutive patients scheduled for carotid artery revascularisation between April 2007 and April 2010. All patients included in this study gave informed consent to undergo the proposed treatment and follow the pre-specified follow-up programme. The ethical committee of our institution was informed about the aims and methods of this study and approved the protocol.
In our centre we have developed a team of experts in vascular medicine to evaluate jointly the most appropriate indication (CAS, CEA or medical treatment alone) in this specific patient setting. The cardiovascular team includes cardiac and vascular surgeons, clinical and interventional cardiologists and neurologists.
Neurologically asymptomatic and symptomatic patients were considered eligible for CAS or CEA if, at duplex ultrasonography, they presented a stenosis involving the internal carotid artery of ≥60% or ≥50%, respectively. Patients were considered to be symptomatic if they had had an ipsilateral cerebrovascular event within 180 days before inclusion. Coronary and cervical cerebral angiography were performed in the majority of neurologically asymptomatic patients, mostly in those with associated or suspected coronary artery disease (CAD) in order to select the therapeutic strategy better6, or in cases of non-diagnostic findings after non-invasive imaging. Patients in need of urgent percutaneous or surgical coronary revascularisation were excluded from the study. After evaluation by the cardiovascular team, the selection procedure for carotid revascularisation (CAS or CEA) was performed within three days.
Fundamental to treatment selection was an understanding of the anatomical findings and medical comorbidities used to categorise patients at high surgical risk, as previously defined in large studies7,8. In these patients, CAS was generally preferred over CEA. Other characteristics were also taken into account, such as complex plaque morphology and aortic arch, severe vessel tortuosity, or small internal carotid artery that were likely better served with CEA1. In case of patients suitable for both procedures (CAS and CEA), CAS was preferred since it is less invasive by nature. Finally, after a detailed description of the advantages and disadvantages of each intervention, the patient’s choice was also taken into account.
CAROTID ARTERY REVASCULARISATION
CEA and CAS were performed according to current recommendations3,4. Briefly, CEA was performed in the usual fashion by using general endotracheal anaesthesia. Patch arterioplasty was the preferred method for arterial reconstruction. Eversion endarterectomy was employed in patients presenting with significant kinking of the internal carotid artery. Selective intraoperative carotid shunting was indicated in case of systolic stump pressure values lower than 50 mmHg and/or drop of cerebral oximetry more than 10% determined by INVOS System (Somanetics Corporation, Troy, MI, USA).
In case of CAS, cerebral protection with filter wires or with proximal occlusion devices was used in all patients. Different types of self-expandable stents were used according to individual clinical and anatomical characteristics, as recommended by experts9.
All patients undergoing either study procedure received medical therapy that was consistent with the current standard of care, including antithrombotic therapy and treatment of hypertension and hyperlipidaemia.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint of the study was the occurrence of major adverse cardiac and cerebrovascular events (MACCE), including any death, non-fatal myocardial infarction (MI) or any stroke at 30 days after carotid revascularisation.
The secondary endpoint was the cumulative incidence of MACCE at long-term follow-up, a composite of any death, any stroke, or MI within 30 days after the intervention or any death or ipsilateral stroke between 31 days and two years.
Deaths were considered irrespective of their aetiology. Fatal stroke (ischaemic or haemorrhagic) and fatal MI were defined as deaths. Non-fatal MI was defined as spontaneous MI, diagnosed by any rise in creatine kinase myocardial band (CK-MB) fraction above the upper limit of normal, in addition to either chest pain or symptoms consistent with ischaemia or ECG evidence of ischaemia, including new ST-segment depression or elevation of more than 1 mm in two or more contiguous leads according to the core laboratory. ECG and cardiac enzymes were routinely assessed before and six to eight hours following both CEA and CAS procedures. Non-fatal stroke was defined as an acute ischaemic neurological event that persisted ≥24 hours, as assessed by a neurologist and confirmed by brain imaging. Strokes were considered disabling (major) if patients had a modified Rankin score of >3 at 30 days after onset of symptoms. A minor stroke was defined as a Rankin score of 3 or less that resolved completely within 30 days. Transient ischaemic attacks and amaurosis fugax were diagnosed if the symptoms disappeared within 24 hours. The neurological status was assessed by a neurologist before and after the intervention in all cases.
DATA COLLECTION AND PATIENT FOLLOW-UP
Patients’ data were entered in a dedicated database. Clinical follow-up was obtained prospectively by either clinical visits or telephonic contacts at 30 days after the intervention and thereafter at six- month intervals.
STATISTICAL ANALYSIS
Continuous variables were reported as mean and standard deviation and were compared using the Student’s t-test or Wilcoxon rank sum test as appropriate. Categorical variables were reported as percentages and compared using the chi-square or Fisher’s exact test as appropriate.
To account for differences in baseline characteristics between the CAS and CEA groups, a propensity analysis approach was used for data analysis. Regression analysis was used to compare baseline characteristics in the two groups, proving the existence of significant differences. A binary logistic regression analysis was then created with surgical versus percutaneous approach as dependent variable and such baseline characteristics as covariates. This produced a continuous three decimal places numeric variable (the propensity score itself). The nearest neighbour matching technique was then implemented to create pairs of cases with matched propensity score and therefore presumably matched baseline characteristics10. If multiple cases were eligible for a best neighbour role, a custom random generation choice algorithm was used to make the choice impartially. At the end of the procedure the baseline characteristics of the two groups were again tested for differences at univariate and multivariate analysis, with no significant differences found.
In the total population and in the matched cohort, univariate analysis to test the relation between the clinical and treatment variables and the occurrence of events included in primary and secondary endpoints was performed by means of binary logistic regression. Subsequent multivariate logistic regression (backward stepwise, remove p≤0.20) including only significant variables at univariate analysis was used to assess the simultaneous effect of multiple variables on the primary endpoint. Multivariable Cox proportional hazards regression (backward stepwise, remove p≤0.20) was used to assess the simultaneous effect of multiple variables on the secondary endpoint. Long-term freedom from MACCE in the total population and in the matched cohort was calculated using the Kaplan-Meier method, and statistical significance was calculated by the Breslow (generalised Wilcoxon) test.
For all analyses, the conventional p-value of 0.05 or less was used to determine the level of statistical significance. All reported p-values are two-sided. All data were analysed using the statistical software package IBM SPSS 20 (IBM, Armonk, NY, USA).
Results
Of the 436 patients selected before admission to our institution for carotid revascularisation between April 2007 and April 2010, 26 were excluded from the analysis for urgent coronary revascularisation and seven for indication to medical treatment alone after cardiovascular team evaluation. Therefore, 403 consecutive patients with COD were included in the present study: 130 were treated with CEA and 273 with CAS. Baseline clinical characteristics of all patients are shown in Table 1.
Patients with symptomatic COD were 115 (28.5%) and the remaining 288 (71.5%) patients presented an asymptomatic COD. Among this latter group, the proportion of patients with at least one high-risk feature was as follows: rapid progression of carotid plaques in 31 (13 receiving CEA and 18 CAS), echo findings of plaque instability in 72 (40 CEA and 32 CAS), severely narrowed (>80%) carotid arteries in 75 (18 CEA and 57 CAS) and clinically silent previous neurologic ischaemic events at cerebral imaging in 81 patients (13 CEA and 68 CAS).
CK-MB levels were collected before CEA or CAS in 98% of patients and at six to eight hours after the procedure in 96%; troponin without CK-MB was obtained in 22.5%, troponin with CK-MB in 15%. An elevation in troponin levels was detected in one patient who underwent CEA, while a rise in CK-MB was present in three patients (two in the CAS and one in the CEA group, respectively).
Eleven baseline variables were used to create a propensity score matching model for selection to CEA versus CAS. The regression analysis for the dependent variable (CEA versus CAS) before and after propensity matching is shown in Table 2.
CAS was successful in 270 patients (98.9%). Cerebral protection was performed in all patients: a filter wire was used in 254 patients (93.0%), and a proximal occlusion device (Mo.Ma system; Invatec, Roncadelle, Italy) in the remaining 19 (7.0%). One stent was implanted in 94.9% of cases, and the remaining 5.1% of carotid lesions treated with CAS required more than one stent. The different types of self-expandable stents used were as follows: RX Acculink™ (Abbott Vascular, Santa Clara, CA, USA) in 40%, Cristallo (Medtronic, Minneapolis, MN, USA) in 3%, PRECISE® (Cordis, Johnson & Johnson, Warren, NJ, USA) in 17%, and WALLSTENT® (Boston Scientific, Natick, MA, USA) in 40% of cases. CEA was successful in all patients. The majority of carotid artery reconstructions after endarterectomy were performed using vein or synthetic patches (111 patients, 85.3%). An eversion endarterectomy was performed in 14.7% of patients, with significant vessel kinking. Temporary intraoperative shunting was required in 36 patients (27.7%).
30-DAY OUTCOME
The incidence of MACCE at 30 days in the study population was 4.0% (95% confidence interval [CI]: 2.1 to 6.0), with no statistically significant difference between the CAS and CEA groups (3.3% versus 5.4%, respectively; p=0.3) (Table 3). At univariate analysis, the incidence of MACCE was higher in patients ≥80 years than in those younger (9.2% versus 2.8%, p=0.009), and in patients with COPD (9.8% versus 3.3%, p=0.04), while it was not statistically different in all other subgroups of patients. Of note, the incidence of any stroke (3.5% versus 2.1%, p=0.4) and any stroke or cardiovascular death (3.5% versus 2.4%, p=0.6) in symptomatic and asymptomatic patients was also similar. Multivariate regression analysis showed that age ≥80 years (OR 3.6, 95% CI: 1.3 to 9.9; p=0.005) and COPD (OR 3.2, 95% CI: 1.0 to 10.3; p=0.03) were the only significant independent predictors of MACCE at 30 days.
In the matched cohort, the incidence of MACCE at 30-day follow-up was 4.8% (95% CI: 2.7 to 6.9), with no statistically significant difference between the CAS and CEA groups (2.2% versus 5.4%, respectively; p=0.24) (Table 4). At univariate analysis, the incidence of MACCE was higher in patients ≥80 years than in those younger (15.8% versus 3.6%, p=0.02) and it reached a borderline statistical difference in patients with symptomatic versus those with asymptomatic COD (10% versus 3.4%, p=0.08). Of note, the incidence of any stroke (2.5% versus 2.1%, p=0.86) and any stroke or cardiovascular death (2.5% versus 2.7%, p=0.93) in symptomatic and asymptomatic patients was also similar.
Among the matched cohort, there was no significant independent predictor of 30-day MACCE at multivariate regression analysis.
LONG-TERM OUTCOME
Follow-up was completed in 98.6% of patients. Median follow-up was 21.5 months (interquartile range 23.5 months), with 289 of the patients (72%) being followed up for at least one year. The cumulative freedom from MACCE at two-year follow-up in our study population was 80.5% ± 0.94%, with no statistically significant difference between the CAS and CEA groups (81.3% versus 80.3%, respectively; HR 1.0, 95% CI: 0.5 to 2.1, p=0.99). The occurrence of the single endpoints included in cumulative MACCE is detailed in Table 5. The Kaplan-Meier curve for two-year freedom from MACCE is shown in Figure 1.
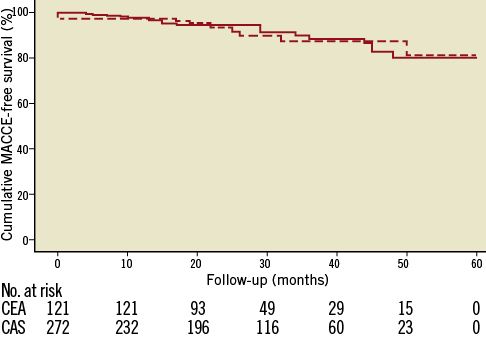
Figure 1. Kaplan-Meier analysis for cumulative survival free of MACCE at long-term follow-up in the total population. The cumulative freedom from MACCE at two-year follow-up was 81.3% and 80.3% in the CAS (dashed line) and CEA (solid line) groups, respectively (p=0.99).
At univariate analysis the incidence of long-term MACCE was higher in patients ≥80 years than in those younger (15.8% versus 5.5%, p<0.002), and in patients with COPD (19.5% versus 6.1%, p<0.002).
Multivariate regression analysis showed that age ≥80 years (HR 3.2, 95% CI: 1.5 to 7.01; p=0.003) and COPD (HR 3.7, 95% CI: 1.5 to 9.1; p=0.003) were the only significant independent predictors of MACCE at two years.
In the matched cohort, the freedom from MACCE at two years proved to be 82.8% ± 1.32% (SE), (90.2% in the CAS and 78.4% in the CEA group; HR 0.84, 95% CI: 0.3 to 2.3, p=0.72). The occurrence of the single endpoints included in cumulative MACCE is detailed in Table 6. The Kaplan-Meier curve for two-year freedom from MACCE is shown in Figure 2.
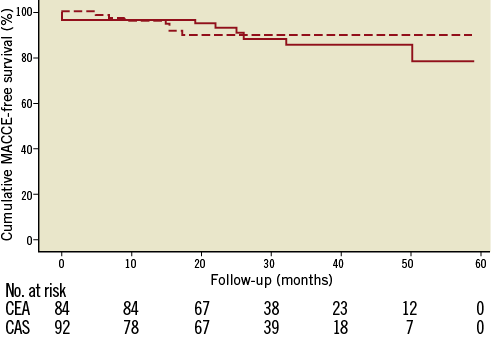
Figure 2. Kaplan-Meier analysis for cumulative survival free of MACCE at long-term follow-up in the matched cohort. The cumulative freedom from MACCE at two-year follow-up was 90.2% and 78.4% in the CAS (dashed line) and CEA (solid line) groups, respectively (p=0.72).
Proportional hazard regression analysis showed that age ≥80 years (HR 2.6, 95% CI: 1.2 to 5.4; p=0.01) and COPD (HR 3.5, 95% CI: 1.5 to 7.9; p=0.003) were the only independent predictors of MACCE at two years.
Discussion
This prospective, real-world registry of patients with COD showed that selection of carotid artery revascularisation by consensus of a cardiovascular team is associated with an acceptable incidence of clinical events at early and long-term follow-up.
To date, the choice of treatment among CEA, CAS, or medical therapy alone for an individual patient with COD remains a controversial issue1. Surgical management of significant COD, as defined historically by the North American Symptomatic Carotid Endarterectomy Trial (NASCET)7 and Asymptomatic Carotid Atherosclerosis Study (ACAS)8, currently remains the gold standard for reducing the risk of a subsequent cerebrovascular accident. However, trade-offs exist when deciding between CEA and CAS. Despite several large, multicentre trials that have been conducted to determine the risks and benefits of each procedure, the literature remains unclear about the absolute benefit of one procedure over the other1. Moreover, the role of CAS is still unproven in patients at average surgical risk, and only a small amount of data on late outcomes from a real-world global population is available. In this setting, clinical judgement and multidisciplinary dialogue remain essential in daily decision making.
Current guidelines strongly recommend that all relevant data should be reviewed by a multidisciplinary panel in order to determine the likelihood of safe and effective revascularisation with either CEA or CAS3,4. In this regard, this is the first published registry to report the global incidence of events after the multidisciplinary consensus of a cardiovascular team.
The large international REACH (REduction of Atherothrombosis for Continued Health) registry, which included more than 3,000 patients with a history of carotid artery revascularisation, indicated that CAS is comparable to CEA, even in propensity score-matched cohorts of patients for late outcomes (up to two years)11. Accordingly, in this prospective registry where treatment strategy was selected by consensus of a cardiovascular team, the freedom from MACCE at two years proved to be superior to 80% and similar in the CAS and CEA groups.
Of note, advanced age was an independent predictor both of 30-day and of long-term MACCE. Thus, in fragile populations, the cost-effectiveness of carotid revascularisation compared to medical treatment alone remains to be established. Interestingly, COPD was also an independent predictor of MACCE, thus suggesting that it should be taken into account in the decision-making process in patients with COD.
In our study, asymptomatic patients were revascularised after cardiovascular team evaluation of patients at higher stroke risk, including those with rapid progression of carotid plaques, echo findings of plaque instability, severely narrowed (>80%) carotid arteries or with clinically silent previous neurologic ischaemic events at cerebral imaging3,4. Specific ongoing randomised clinical trials will clarify the role of carotid revascularisation in patients with asymptomatic carotid artery stenosis, at least in those at higher risk. Nevertheless, the 30-day stroke rate in our asymptomatic patients was 2.2% (matched cohort), inferior to the recommended cut-off level of 3%3,4.
Our data obtained in an all-comers, real-world population seem to be even better compared to the long-term findings of the SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) trial that focused on patients, both symptomatic and asymptomatic, at high risk for surgery treated with CEA or CAS performed by operators with minimum recommended experience and with the systematic use of embolic protection devices12. In the latter study MACCE at one year occurred in 12.2% in the CAS group and in 20.1% in the CEA group (p=0.053), with the difference mainly driven by a reduction in the rate of MI12. Accordingly, recent data from the Carotid Revascularization Endarterectomy versus Stent Trial (CREST), a large randomised study that included both patients with symptomatic and those with asymptomatic COD at various levels of surgical risk, demonstrated an increased risk of stroke among patients undergoing CAS compared with CEA (4.1% versus 2.3%) and a decreased risk of MI in CAS compared with CEA (1.1% versus 2.3%), leading to similar outcomes when combining death, MI, and cerebrovascular events2. Compared to these studies2,12, the lower incidence of early and long-term MACCE and the similar rates of MI and stroke in the CAS and CEA groups observed in our study are probably due to an appropriate selection of carotid revascularisation, together with the high experience and individual, tailor-made approach employed in our centre.
In addition, the technology used with CAS has advanced tremendously during the past decade, including increased familiarity of practitioners with the technique and the advent of new stents and distal protection devices making randomised controlled trials studies of the past, rather than of the present. CREST, in particular, used one distal protection device which has subsequently been modified2. In this regard, several CAS consensus documents have focused on the certification of centres performing carotid revascularisation, in terms of specific training not limited to catheter skills but also including all aspects of carotid disease management, and on a tailored approach, using appropriate stents and neuroprotection devices based on plaque morphology and carotid anatomy5.
Study limitations
The decision regarding therapeutic strategy was based on the clinical judgement and agreement of the cardiovascular team. The low MACCE rates observed in this study could be related not only to patient selection (based on multidisciplinary decision making), but also to operators’ skill in a single high-volume institution and the possibility of performing individual, tailor-made procedures, and therefore they cannot be extrapolated to all centres. An objective preoperative method or score to stratify the pre-interventional neurological or cardiac outcomes of patients undergoing CAS or CEA independently is lacking and has not been used. In addition, events were reported by the investigators, and there was no central adjudication of clinical events.
Conclusions
In this real-world registry of consecutive patients undergoing carotid artery revascularisation selected by consensus of a cardiovascular team, the early and long-term incidence of clinical events is valuable. Further long-term studies are required to define the impact on outcomes of multidisciplinary decision making and the tailored approach in both CAS and CEA.
| Impact on daily practice Most of the randomised clinical trials comparing carotid artery stenting (CAS) and carotid endarterectomy (CEA) present several methodological limitations and provide controversial results. In order to identify the most appropriate treatment for each single patient with carotid obstructive disease, we showed that early and long-term outcome in an all-comers population of patients undergoing CAS or CEA selected by consensus of a cardiovascular team is valuable. Thus, clinical judgement and multidisciplinary dialogue need to be considered in daily decision making. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

