
Introduction
Carotid artery stenting (CAS) is associated with a stroke risk, mainly due to dislodgement of debris from the target lesion during the procedure. The stent’s scaffolding capacity plays a major role in preventing procedural events1. The introduction of dual-layer stents might reduce the periprocedural risk of embolisation by extensive plaque coverage and prevention of plaque prolapse.
The CLEAR-ROAD trial evaluated the clinical outcomes of treatment with the Roadsaver carotid artery stent in subjects at high risk for carotid endarterectomy requiring carotid revascularisation due to significant extracranial carotid artery stenosis. This report presents the 12-month outcomes of all 100 included patients.
Methods
STUDY DESIGN
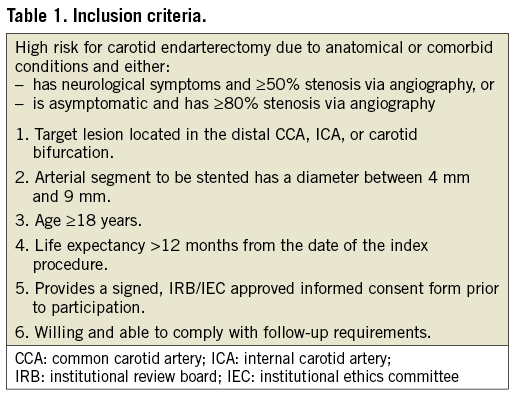
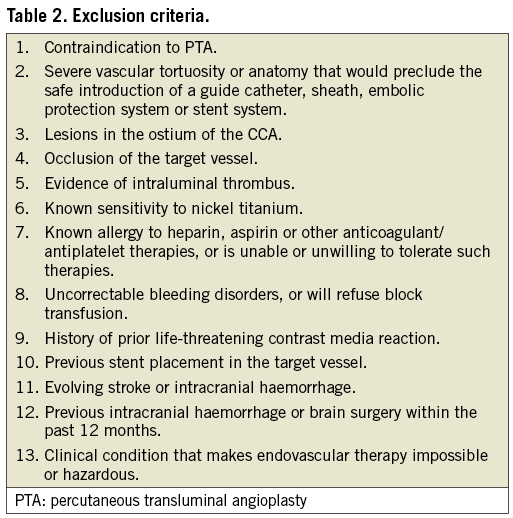
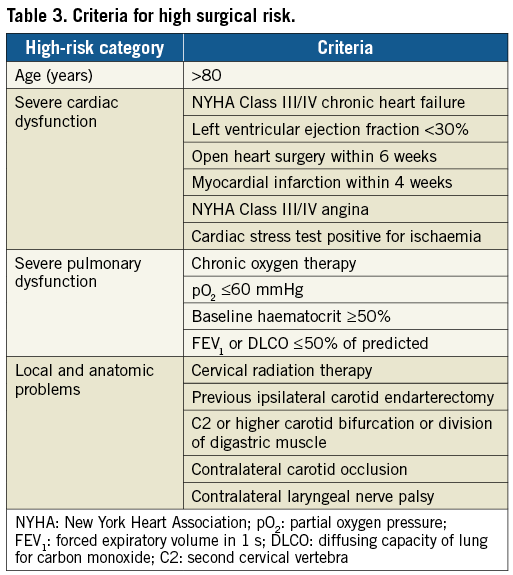
The CLEAR-ROAD study was a prospective, multinational, single-arm study in 100 patients. An overview with enrolment per centre is shown in Figure 1. The inclusion/exclusion criteria are described in Table 1 and Table 2. The criteria for high surgical risk are listed in Table 3. A patient was considered to be at high surgical risk if one of those criteria was fulfilled. Neurological evaluation was conducted by an independent individual certified to perform NIH Stroke Scale assessment before procedure, at discharge, and at 30-day, 6-month and 12-month follow-up visits.
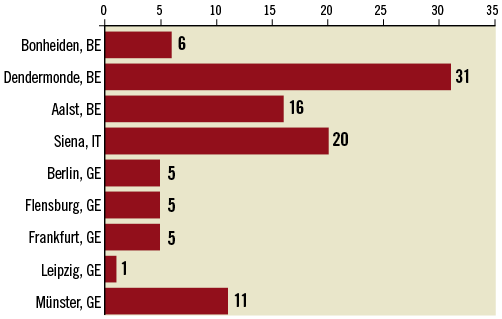
Figure 1. Overview with enrolment per centre.
PROCEDURE
The CAS procedure was performed according to the physician’s standard of care and based on the instructions for use of the Roadsaver® carotid artery stent system (Terumo Corp., Tokyo, Japan). Use of an embolic protection device (EPD) was not mandatory and some investigators felt more confident not using an EPD with this type of stent. Concomitant medication during hospital stay and follow-up was recommended to be clopidogrel 75 mg daily for one month and aspirin 75-300 mg daily lifelong.
FOLLOW-UP
All participants were asked to visit centres at 30 days, 6 months and 12 months after the index procedure. At each visit, carotid duplex ultrasound (DUS), neurological assessment (NIHSS), WW medication registration, physical examination and adverse event recording were routinely conducted.
Results
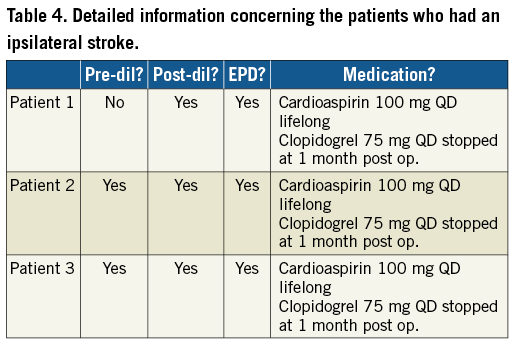
Demographics, procedural details and data until 30 days post procedure have been fully described in an earlier article2. This manuscript will focus on the long-term data from the period between day 31 and 12 months post procedure. Between day 31 and day 365, three patients suffered an ipsilateral stroke, resulting in a 12-month rate of freedom from ipsilateral stroke of 95.80%. More detailed information on medication and procedural characteristics for the patients who had an ipsilateral stroke can be found in Table 4.
PATENCY AND TARGET LESION REVASCULARISATION RATES
Standard DUS was performed by the investigators. Percentage of stenosis was determined according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) guidelines3. At 12-month follow-up, the primary patency rate was 92.50% and the freedom from target lesion revascularisation rate was 97.90%.
NEUROLOGICAL ASSESSMENT
Neurological assessment was carried out using the standardised NIHSS classification. The evolution in stroke classification over the different timepoints is displayed in Figure 2.
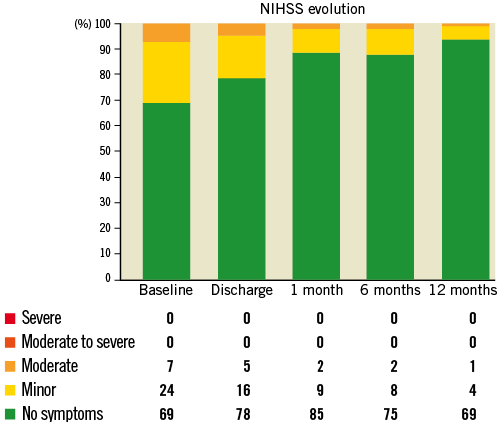
Figure 2. Evolution in stroke classification.
Discussion
The rate of 12-month ipsilateral stroke is the most appropriate variable to assess the efficacy of CAS. In the ICSS trial the rate of procedural stroke or ipsilateral stroke was 9% in the CAS arm versus 4.7% in the carotid endarterectomy (CEA) arm at one year. In the CREST trial the rate of ipsilateral stroke at one year was 6% in the CAS group versus 2.8% in the CEA arm. The CLEAR-ROAD trial showed a freedom from ipsilateral stroke rate of 95.8% at 12 months, meaning that the treatment of a carotid stenosis with the Roadsaver stent is effective in preventing long-term (one-year) ipsilateral stroke.
Carotid restenosis and the need for repeat revascularisation (target lesion revascularisation [TLR]) are also important long-term properties. In the CREST trial for example, the restenosis rate after two years based on peak systolic velocity (PSV) greater than 3 m/s was 6.3% in the CAS arm versus 6.0% in the CEA arm. In the SPACE trial, after two years the restenosis rate in the CAS arm was 10.7%. This means that the results of the CLEAR-ROAD trial with a freedom from restenosis rate of 92.5% and freedom from TLR rate of 97.9% are in line with the reported restenosis rates in previous CAS trials.
Limitations
A limitation was the small number of study patients in this non-randomised study.
Conclusions
The short- and long-term clinical outcome of 100 patients treated with a dual-layer micromesh carotid stent (Roadsaver) shows promising results. The Roadsaver stent is a safe and effective device for endovascular treatment of subjects at high risk for carotid endarterectomy.
| Impact on daily practice The Roadsaver stent is a valid alternative to treat carotid artery lesions and could be incorporated into daily practice. |
Funding
This was an investigator-initiated trial, funded by Terumo.
Conflict of interest statement
D. Scheinert: advisory board/consultant for Abbott, Biotronik, Boston Scientific, Cook Medical, Cordis, CR Bard, Gardia Medical, Medtronic/Covidien, TriReme Medical, Trivascular, Upstream Peripheral Technologies. H. Sievert: study honoraria, travel expenses, consulting fees from Abbott, Acoredis, Atrium, Biosense Webster, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, CardioKinetix, Celonova, CGuard, Coherex, Comed B.V., Contego, CSI, CVRx, ev3, FlowCardia, Gardia, Gore, GTIMD Medical, Guided Delivery Systems, Hemoteq, InspireMD, Kona Medical, Lumen Biomedical, Lifetech, Medtronic, Occlutech, PPM Medical, Record, SentreHeart, Svelte Medical Systems, Terumo, Trivascular, Valtech, Vascular Dynamics, Venus Medical, Veryan, Cardiokinetix, Access Closure, Coherex, and SMT. S. Müller-Hülsbeck: speaker fees from Terumo Europe, and grants for Terumo Carotid workshops. The other authors have no conflicts of interest to declare.

