Abstract
Aims: This multicentre, randomised, double-blind study compared the nephrotoxicity of low-osmolar, low-viscous iopromide and iso-osmolar, high-viscous iodixanol in Chinese patients with moderate renal dysfunction, after coronary angiography or percutaneous coronary intervention (PCI).
Methods and results: The primary endpoint was contrast-induced nephropathy (CIN) on day 3, defined as a post-dose increase in serum creatinine (SCr) of ≥50% from baseline. All patients were rigorously hydrated from six hours before intervention. In 562 evaluable patients (of 592 recruited), the contrast volume, presence of diabetes mellitus, mean baseline SCr and estimated glomerular filtration rate (eGFR) were comparable between the iopromide- and iodixanol-treated groups. SCr increases of ≥50% occurred in 1/278 (0.4%) of patients after iopromide and 1/284 (0.3%) after iodixanol. Incidences in the secondary endpoints were the following: SCr increases of ≥0.5 mg/dL, 1.4% and 0.7%, respectively; SCr increases of ≥25%, 5.4% and 2.8%; eGFR decreases of ≥25%, 3.6% and 2.5%. Only one patient showed renal failure, one week after dosing with iodixanol. All differences were statistically insignificant, in the overall collective group and in the subgroup with diabetes (n=170).
Conclusions: With rigorous hydration, the CIN incidence was very low in patients with moderate renal dysfunction who underwent coronary angiography or PCI. No difference in nephrotoxicity was found between iopromide and iodixanol.
Introduction
Contrast-induced nephropathy (CIN; also referred to as contrast-induced acute kidney injury [CI-AKI]) is an acute impairment of renal function occurring after the administration of contrast-enhancement agents in the absence of other aetiologies1,2. CIN is a major complication of interventional coronary procedures, increasing morbidity and prolonging hospitalisation3. The number of cardiac angiography and percutaneous coronary interventions (PCI) has increased steadily in recent years4. Chronic kidney disease is the most important risk factor for CIN5-8. Modifiable risk factors for CIN include hydration (volume supplementation) status, the avoidance of first-generation ionic (“high-osmolar”) contrast agents, the amount of contrast agent, use of concomitant nephrotoxic agents and recent administration of contrast agent7,9-11. Despite numerous studies and meta-analyses12,13, the propensities of the various contrast agents to cause CIN have not been definitively established.
The NEPHRIC study14 engendered the hypothesis, based on 129 cardiac-catheterisation patients, that iso-osmolar contrast agents are associated with better renal tolerance in patients with impaired renal function than low-osmolar contrast agents. The CARE study15 concluded that the rate of contrast-induced nephropathy was not statistically different after the administration of low-osmolar iopamidol or iso-osmolar iodixanol in high-risk patients, and that any difference between the agents was unlikely to be clinically significant. Similar results were obtained for the low-osmolar agents, ioversol and iopromide in other multicentre studies16,17.
In China, the number of cardiac catheterisation procedures is rising dramatically, with over 290,000 procedures in 2011. China now ranks number three in PCI procedures after the USA and Germany18, but information regarding the prevalence of high-risk patients undergoing coronary angiography and regarding the incidence of CIN in high-risk patients who receive comprehensive prophylactic measures (such as rigorous hydration) is scarce. Results of single-centre investigations have been contradictory19,20. It was considered desirable to address these questions in an appropriate study.
We therefore conducted a large-scale, multicentre study to compare the nephrotoxicities of iopromide 370 and iodixanol 320 in Chinese patients with moderate renal dysfunction after coronary angiography or PCI. Additionally, we investigated the prevalence of baseline renal impairment among Chinese patients undergoing elective cardiac catheterisation.
Methods
The DIRECT study was a prospective, multicentre, randomised, double-blind, parallel group comparison of iopromide 370 and iodixanol 320 conducted at 19 centres in China between February 2009 and November 2010 (Clinical Trial Registration NCT00926562). The study was approved by the Institutional Review Board of each centre and performed in conformity with good clinical practice and the Declaration of Helsinki. Written informed consent was provided by all patients before enrolment.
PREVALENCE OF RENAL IMPAIRMENT AMONG PATIENTS UNDERGOING ELECTIVE CARDIAC CATHETERISATION
Initial recruitment rates were lower than anticipated. Therefore, between 1 June 2009 and 30 June 2010, we also investigated the prevalence of baseline renal function among 7,976 consecutive screened patients at 11 study centres. The eGFR calculated from the serum creatinine (SCr) level as an index of renal function was determined before administration of a contrast agent, using the abbreviated modification of diet in renal disease (MDRD) formula21. This was based on National Kidney Foundation definitions5: severe, eGFR <30 mL/min/1.73 m2; moderate, 30 ≤ eGFR <60 mL/min/1.73 m2; mild, 60 ≤ eGFR <90 mL/min/1.73 m2.
PATIENTS
Adult (≥18 years) Chinese patients of both sexes scheduled to undergo diagnostic cardiac angiography or elective PCI were screened. The principal inclusion criterion was moderate renal dysfunction (see above); patients with severe renal dysfunction were not included. Other principal exclusion criteria were pregnancy, lactation, intra-arterial or intravenous administration of an iodinated contrast agent from seven days before to 72 hours after the administration of a contrast agent, intake of any nephrotoxic medications 24 hours before or after the administration of a contrast agent, left ventricular ejection fraction (LVEF) <30% by ultrasound examination, cardiogenic shock, or other contraindications.
STUDY PROTOCOL
Patients included were randomised in equal numbers to receive iopromide (Ultravist, 370 mg I/ml; Bayer Healthcare, Berlin, Germany) or iodixanol (Visipaque, 320 mg I/ml; GE Healthcare, Chalfont St. Giles, UK) and underwent cardiac catheterisation. Double-blinding (up to database lock) was maintained by having the preparation, dispensation, administration and accounting of the contrast agents performed by a physician other than the investigator. Likewise, eligibility was assessed by physicians who did not participate in further study assessments before the blind was broken.
Procedures and interventions were performed according to standard practice at the study sites. The contrast agent was administered intra-arterially. The volume of contrast agent was determined by the patient’s age, weight, clinical indication and examination technique. The volume of contrast agent was thus not standardised, and iodine concentration differences between iopromide 370 and iodixanol 320 were not adjusted.
Patients received prophylactic intravenous hydration with 500 ml 0.9% saline over at least the six hours up to the cardiac catheterisation procedure, followed by a further 1,000 ml after the start of the procedure; the rate of administration was 1-1.5 mL/kg per hour throughout. The hydration regimen was determined in advance by consensus of the responsible physicians, in relation to institutional practice and standard guidelines. Additional pharmacological prophylaxis (e.g., N-acetylcysteine) was not required.
Blood samples for baseline SCr and eGFR determination were obtained before hydration and again 72±12 hours after dosing (or as closely to that as allowed by the patient’s treatment regimen), to be repeated on day 7 if the SCr level increased to >0.5 mg/dL or by >25%. All SCr samples were analysed centrally.
Each patient was contacted by telephone 30 days after administration of the contrast agent, to ask whether hospitalisation, dialysis, any treatment(s) for acute renal failure, or death had occurred, and to record any adverse events.
ENDPOINTS
The primary endpoint was the incidence of relative increase in SCr of ≥50% from baseline to 72 hours after study agent administration. Non-inferiority of iopromide was tested for (see below). Secondary endpoints were a post-dose SCr increase of ≥25%, a post-dose SCr increase of ≥0.5 mg/dL, a post-dose eGFR decrease of ≥25% and the rate of renal failure 30 days post treatment. Adverse events were assessed.
STATISTICAL ANALYSIS
The sample size for this non-inferiority study was based on the proportion of patients with an SCr increase of ≥50%. This proportion was assumed to be 5% in both treatment groups. The non-inferiority margin was set at 5.5% by a combination of statistical reasoning and clinical judgement. It was estimated that with at least 80% power and a significance level of 0.025 (one-sided), a total sample size of 564 patients (282 patients per group) would be required to determine non-inferiority of iopromide. The sample size estimation was based on a one-sided Z-test with continuity correction (pooled). To allow for ~5% dropouts or non-evaluable patients, enrolment of a total of 590 patients was planned.
Data are presented as percentages or as mean±standard deviation (SD). Comparison of baseline data was performed using the χ2 test or Fisher’s exact test (categorical variables) and the Student t-test (continuous variables).
Eligibility for CIN analysis was prospectively defined to include patients who received a randomised contrast agent, underwent only one cardiac catheterisation procedure during the study period, had SCr measurements at baseline and 24-120 hours after dosing, and for whom no protocol violations (failure to meet inclusion criteria, meeting exclusion criteria, >1 angiographic procedure from the day of study treatment to the last study creatinine measurement, no cardiac catheterisation after randomisation, additional contrast medium, a critical clinical event during follow-up) occurred. Critical clinical events were defined as those very likely to compromise renal function (e.g., cardiac arrest, acute myocardial infarction, cardiovascular collapse, shock, or major surgery).
The difference in the incidences of CIN defined as an SCr increase of ≥50% (primary endpoint) were analysed by a Wald test for the risk difference on non-inferiority of iopromide (in this analysis “inferiority” is understood as a higher rate of a given potentially adverse safety result). In addition a superiority test was performed as a secondary analysis for primary and secondary definitions of CIN. One-sided p-values <0.025 were to be considered statistically significant in non-inferiority testing. In all other tests two-sided p-values <0.05 were to be considered statistically significant. The statistical analysis employed SAS version 9.13 (SAS Institute, Cary, NC, USA).
A literature search of prospective randomised trials comparing low-osmolar contrast agents with iso-osmolar iodixanol in renally compromised patients undergoing cardiac catheterisation was performed. All studies that met the search criteria starting from the publication of NEPHRIC in 2003 were included.
Results
Between February 2009 and November 2010, ~24,000 PCI candidate patients were surveyed/screened at centres in China.
PREVALENCE OF RENAL IMPAIRMENT IN CHINESE PATIENTS UNDERGOING CARDIAC CATHETERISATION (baseline survey)
A baseline survey of 7,976 patients who underwent elective coronary angiography or PCI at 11 study centres revealed that the prevalence of normal and mild renal dysfunction was 50.67% and 40.95%, while the prevalence of moderate and severe renal insufficiency (eGFR of <60 mL/min/1.73 m2) was 8.38% (moderate: 7.45%; severe: 0.93%; Figure 1). The patients with moderate renal impairment therefore accounted for about 90% of those at high risk of CIN (eGFR of <60 mL/min/1.73 m2).

Figure 1. Prevalence of renal insufficiency among patients who underwent elective cardiac catheterisation (baseline survey; n=7,976).
STUDY POPULATION
At 19 centres, 592 patients (6-84 per centre: median 31) were eligible and were enrolled in the study (Figure 2).

Figure 2. Flow diagram for patients in the study.
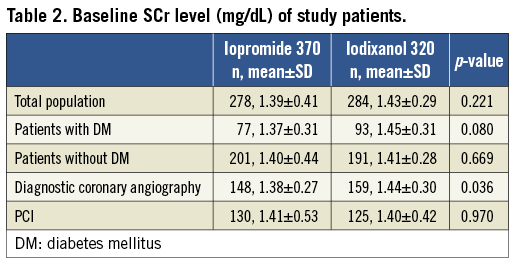
Of the 562 evaluable patients, 278 received iopromide and 284 received iodixanol. The demographic, clinical, and procedural characteristics of the patients are shown in Table 1. The groups were comparable with regard to gender and race distribution, presence of diabetes mellitus, time-point of post-dose sampling, distribution by type of procedure (diagnostic cardiac angiography or PCI). Baseline eGFR was also comparable: 49.56±8.10 mL/min per 1.73 m2 in the iopromide group and 48.53±11.86 mL/ min per 1.73 m2 in the iodixanol group (p=0.23). Details of pre-dose SCr are reported in Table 2. Patients who received iopromide received a larger volume of contrast agent. As iopromide 370 is also more concentrated than iodixanol 320, this resulted in a ~30% higher amount of iodine received by the patients in the iopromide group. The distribution of times of sampling for SCr was similar to that reported for the CARE study15.
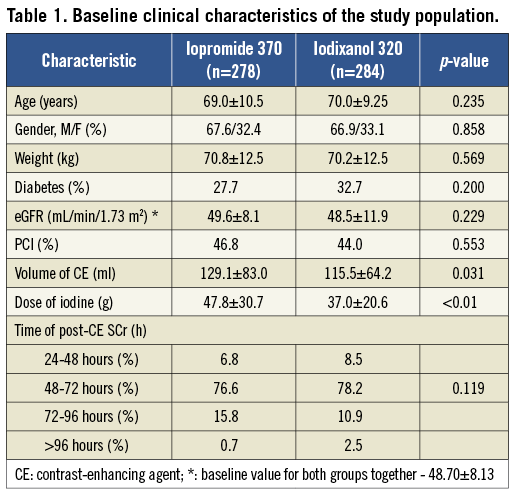
INCIDENCE OF CIN
The incidence of CIN did not differ statistically between the groups (Table 3), irrespective of the definition used (see “endpoints”). Only one patient in each group had an increase in SCr from baseline of ≥50%, which was the prospectively defined primary endpoint. The incidences of CIN were 0.4% in the iopromide 370 group (1/278 patients) and 0.4% in the iodixanol 320 group (1/284 patients); the 95% confidence interval for the difference was -1.0% to +1.0%. Thus, non-inferiority of low-osmolar iopromide to iso-osmolar iodixanol was demonstrated, with p<0.001 (Table 3).
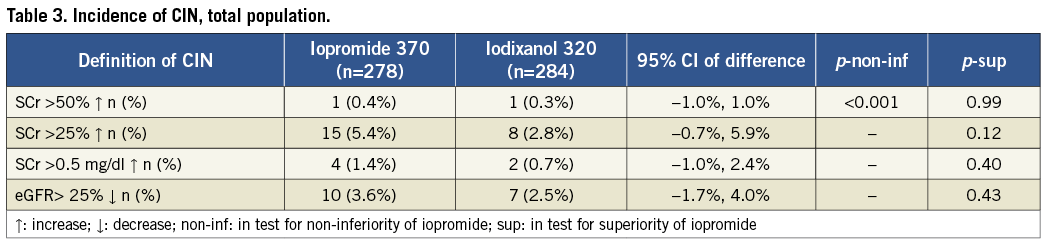
In terms of the secondary endpoints, the incidence of an SCr increase of ≥0.5 mg/dL was close to 1% in both groups (Table 3), while that of an SCr increase of ≥25% was higher in both groups (5.4% for iopromide 370 and 2.8% for iodixanol 320). Rates of eGFR decreases of ≥25% were similar (Table 3).
The average dose of contrast agent (in ml) was three times below the eGFR (in mL/min/1.73 m2; Table 1), a value previously cited as critical22,23. To look for any possible dose effect, we compared the rates of CIN among patients for whom this ratio was >3 and ≤3. Among 222 patients with a ratio >3, six (2.7%) experienced CIN; of the 340 patients with a ratio ≤3, 19 (5.6%) also did so. Thus, a higher rate of CIN accompanying a higher ratio of contrast agent volume to eGFR was not observed.
SUBGROUP ANALYSIS
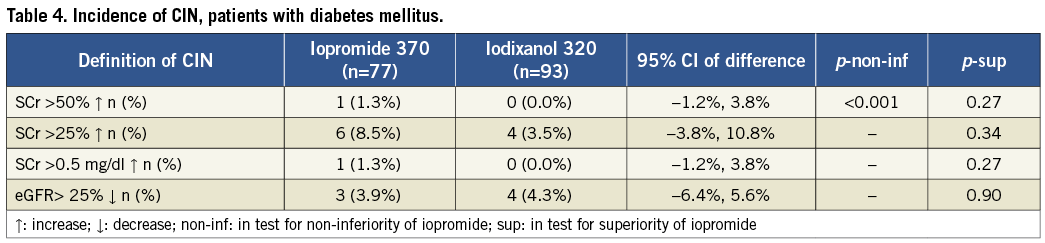
CIN occurrence in patients with diabetes mellitus (the NEPHRIC population14) also showed no significant difference between the groups, regardless of the CIN endpoint (Table 4). In 170 diabetic patients, an SCr increase of ≥50% occurred in 1.3% after iopromide (1/77 patients) and 0% after iodixanol (0/93; p=0.27). Again, non-inferiority of iopromide was demonstrated. For the secondary endpoints: respective SCr increases of ≥25% were 8.5% and 3.5% (p=0.34), SCr increases of ≥0.5 mg/dL were 1.3% and 0% (p=0.27), rates of eGFR decreases of ≥25% were 3.9% and 4.3% (p=0.90).
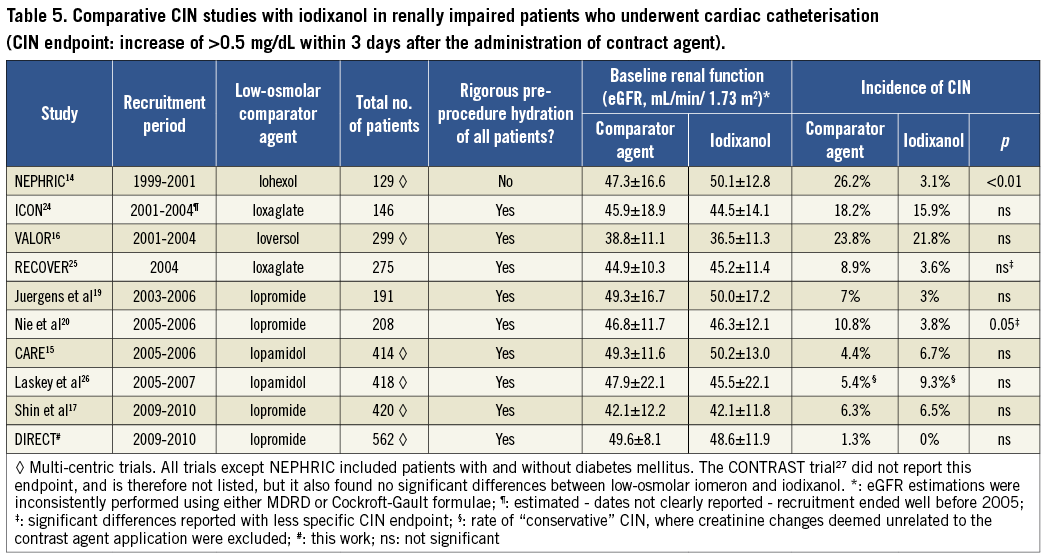
Owing to the low CIN rate, no additional subgroup analyses were performed. Our results are compared with those of published prospective comparative trials in Table 5.
MEAN PEAK CHANGES IN SCR LEVEL
Mean peak changes in SCr seemed to favour iopromide, but no significant difference was found between iopromide and iodixanol in the total population (–3.38±34.27 versus –2.37±17.844, p=0.6589) or in patients with diabetes mellitus (–0.60±18.997 versus 1.31±17.389, p=0.4940; Figure 3). An important observation was that the mean post-dose SCr levels decreased compared with mean pre-dose SCr levels, both in the total population and in diabetic patients receiving iopromide, whereas the mean post-dose SCr level was increased in diabetic patients after iodixanol exposure.
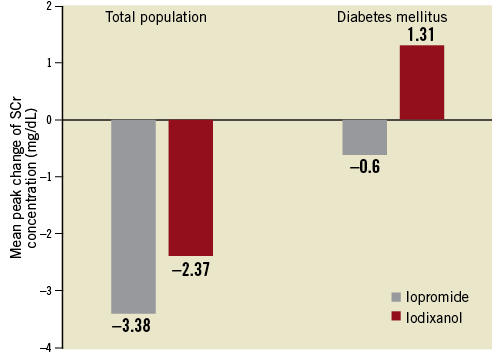
Figure 3. Serum creatinine: mean peak changes (mg/dL) from baseline.
RENAL FAILURE DURING 30-DAY FOLLOW-UP
No patient required haemodialysis; no study-related deaths were reported. Only one patient in the iodixanol group, who was not diagnosed with CIN on day 3, was found to have an SCr level that increased by ≥25% one week after discharge from hospital. During a one-year post-treatment follow-up his overall condition was stable with his SCr level remaining high at around 2.26 mg/dL.
NON-RENAL ADVERSE EVENTS
Adverse events were recorded for 50 of the 592 patients (8.4%), 30 (10.3%) in the iopromide group and 20 (6.7%) in the iodixanol group (p=0.1392). Most (10.3% and 6.7%, respectively) of these were non-serious and resolved spontaneously. In the iodixanol group one patient died after surgery, which was considered by the investigator to be unrelated to the study agent.
Discussion
Our study has revealed that, in the population of Chinese patients with moderate renal dysfunction, undergoing cardiac catheterisation and with rigorous hydration, CIN was very infrequent. We found no significant difference in its occurrence between those receiving the low-osmolar, non-ionic monomer iopromide 370 and those receiving the iso-osmolar dimer iodixanol 320. Non-inferiority of iopromide compared with iodixanol was demonstrated. During the 30-day follow-up, no patient required haemodialysis. A survey conducted in 11 of the 19 study centres showed that the prevalence of moderate and severe renal insufficiency among patients undergoing coronary angiography and elective PCI were 7.45% and 0.93%, respectively, and thus lower than anticipated.
The primary endpoint used –changes in SCr– has been used as a surrogate parameter in renal function safety trials14,15,25. Less specific thresholds of the surrogate definition of CIN may be prone to spurious effects unrelated to contrast application28, so the threshold for “renal risk” (the incidence of relative increase in SCr by ≥50%, as defined by the RIFLE criteria developed by the Acute Dialysis Quality Initiative (ADQI) group for the nephrological scientific community29,30) was used as the primary endpoint in this study.
Secondary endpoints were chosen for comparability with earlier CIN studies in this area14,15,25. Criteria to be considered include clinical importance, responsiveness to the intervention, precision definition, and accuracy and feasibility of measurement29. None of the surrogate endpoints has been validated prospectively against hard clinical endpoints; association between post-procedural SCr increases in renal risk patients and morbidity/mortality has been seen in large cardiological registries8. It remains unclear which definition of post-procedural SCr changes is the most appropriate to describe the risk for the patients.
However, the two major findings of this study are unaffected by the choice of endpoint: rates of CIN were low, and they did not differ statistically between the groups (iopromide: 0.4%; iodixanol: 0.3%). This absence of a significant difference was found throughout the study. This corresponds to earlier data on the renal tolerance of low-osmolar compared with iso-osmolar contrast agents. Table 5 lists all published randomised comparative trials, and shows clearly that the NEPHRIC hypothesis could not be confirmed. This may be ascribed to the limitations of the NEPHRIC study (small sample size, baseline imbalance, inconsistent hydration of the patients). Our results are in line with the other data, and with 562 patients this study is to date the largest prospective, randomised, double-blind comparison of nephrotoxicity between contrast agents in high-risk patients undergoing cardiac catheterisation.
The rates of post-procedural SCr increase that we observed are lower than in earlier trials, although this difference is not great (Table 5). Using the less sensitive increase of ≥0.5 mg/dL listed in Table 5, the rates found in this study are not far from the lowest rates seen in other trials (rates of 3-4% have been reported in several trials). A 50% increase in SCr was not reported in most of the other trials, but so-called “severe CIN” rates, such as increases of 1.0 mg/dL, have frequently been investigated and have been shown to have a very low rate as well (e.g., 0% for iodixanol in NEPHRIC and 2.9% for all patients by Shin et al). If the threshold defining the SCr increase is lowered, then the calculated incidence rates in all studies become higher, but again the rates we found are not much lower than those lowest rates reported from other studies. The low rates may be explained by study-specific factors such as the more homogenous study population and, especially, hydration (all the patients in our study received rigorous, prolonged hydration, with appropriate caution in older patients or in those with known reduced LVEF); also, the present study population was less severely impaired, consistent with the lower incidence of CIN that we observed14,15,19,25,26.
A surprising observation was the difference in contrast agent volume between the treatment groups (Table 1). However, as stated above, this was not fixed by the study protocol, but was determined by local standard procedures, so we infer that it was a random effect. Importantly, the volume of study drug was greater than that of control drug; consequently, the conclusion of the study is not affected, as the overall exposure to the study drug was greater.
We conclude that it is reasonable to infer that the tendency to develop CIN is very low for most high-renal risk patients given low-osmolar contrast media (LOCM) or iso-osmolar contrast media (IOCM) for elective coronary angiography or PCI, as long as they receive rigorous hydration. This conclusion is supported by a recent review that proposes possible mechanisms of contrast agent-related kidney injury2.
Our baseline survey showed that the prevalence of moderate and severe renal insufficiency was respectively 7.45% and 0.93% among Chinese patients undergoing coronary angiography and elective PCI, which is lower than had been anticipated. Patients with moderately impaired renal dysfunction accounted for ~90% of the renal-risk population.
The study’s most important limitation is that the incidence rates assumed for the primary endpoint were anticipated to be 5%, which proved to be an overestimation. Of the secondary endpoints, only the incidence of an SCr increase of >25% in the iopromide group (5.4%) was close to 5%. Thus, robustly adequate powering would have required a study at least three times larger (even though this study was the largest of its kind to date). Nevertheless, the primary objective (to demonstrate non-inferiority of iopromide over iodixanol) was achieved.
A second limitation is that a measurement of SCr on day 7 was available for only 8.5% of the study patients (48/562). This was because, following clinical routine, most patients were discharged on day 3 and did not return to the hospital on day 7. Of the 48 patients with an SCr increase of >25% on day 7, four did not show an SCr increase of >25% on day 3. This accords with other reports that SCr may not peak until 4-5 days after contrast agent administration31. Consequently, future CIN trials of this kind should include measurements on day 7, or even later, after contrast agent administration.
One strength of our study is its relatively large and homogenous study population, yielding a robust comparability of the two study groups and reducing the probability of spurious effects. We see no reason to suppose that our conclusion could not be generalised to patients of any ethnicity. A repetition with severely renally impaired patients would be desirable, but probably impracticable given the logistical, ethical and cost hurdles.
Conclusions
The DIRECT study is to date the largest prospective randomised, double-blind comparison of the nephrotoxicity between different contrast-enhancement agents. It focused on patients at high risk of renal complications after cardiac catheterisation. The results demonstrate that the patients with moderate renal dysfunction account for most of the high-risk patients for CIN, and that adhering to a rigorous hydration regimen in patients at risk results in a very low incidence of CIN as revealed by relevant changes in SCr level. The nephrotoxicity of iopromide was found not to be statistically different from that of iodixanol 320. The attention of the treating physician can therefore shift from the question of the choice of contrast or the type of prophylaxis towards focusing on the actual core task, i.e., performing a successful cardiac catheterisation even in a patient at high risk of renal complications.
Acknowledgements
We are grateful to all the study investigators, coordinators, and patients who participated in the DIRECT study. We thank Dr. Philipp Lengsfeld (Bayer Healthcare, Germany) for a thorough review of the manuscript. We are grateful to all the patients who participated in the DIRECT study. We thank Zhaohui Wei, PhD, for her contribution to the statistical analysis in this study. We also thank the other investigators and their staff who participated in the DIRECT study, including: Zhijun Sun; Hongbin Liu; Lian Chen; Zhifeng Wang; Xiaofang Gu; Xiaoying Liang; Qinhua Jin; Wei Dong; Zhenghong Fu; Yihong Ren; Fusui Ji; Wenduo Zhang; Hui Li; Guisong Wang; Dapeng Zhang; Ying Liu; Xiaosha Wang; Honglei Ji; Huan Sun; Yingyi Zhang; Ru Zhao; Mingchang Li; He Huang; Meixiang Xiang; Jianjing Lin; Xinqun Hu; Ping Huang; Fangqing Niu; Guowei Zhou; Bili Zhang; Guohong Chen; Wei Tian; Tianbao Yao; Linghong Shen.
Funding sources
This work was supported by an unrestricted research grant (no. 14147) from Bayer Healthcare, Germany, and supported in part by grants from Chinese High-Tech (863 Programme, no. 2009AA02Z420), the National Natural Science Foundation of China (no. 81070185) and the China Postdoctoral Science Foundation (no. 201003776).
Conflict of interest statement
Y. Chen has received speaker’s expenses from Bayer Healthcare. All of the other authors have no conflicts of interest to declare.

