Abstract
Aims: Paravalvular aortic regurgitation (PVAR) after balloon-expandable transcatheter aortic valve implantation (TAVI) remains difficult to quantify, and the utility of the AR index (ARi) to create a composite aortic insufficiency (CAI) score was an important advance. Heart rate (HR) influences the ARi but the clinical relevance of this phenomenon remains poorly appreciated. We sought to validate a new composite heart-rate-adjusted haemodynamic-echocardiographic aortic insufficiency (CHAI) score in the prognostic evaluation of PVAR after balloon-expandable TAVI.
Methods and results: The severity of PVAR was assessed immediately post TAVI by transoesophageal echocardiography (TOE) with simultaneous assessment of transcatheter haemodynamics. A total of 303 patients were studied. The CHAI score, incorporating the HR-adjusted diastolic-delta (HRA-DD, the difference between left ventricular and aortic diastolic pressures/HR*80), had a greater discriminatory value for one-year mortality than both PVAR by TOE (p=0.0018) and the previously proposed CAI score, based on the ARi without HR adjustment (p=0.0029). The CHAI score also better stratified percentage increases in left ventricular systolic chamber dimensions at one month and serum natriuretic peptide levels at one to three months.
Conclusions: Prognostication of PVAR in the intermediate range of echocardiographic severity remains unreliable and is greatly enhanced by the integration of heart-rate-adjusted transcatheter haemodynamics.
Abbreviations
AI: aortic insufficiency
AoDBP: aortic diastolic blood pressure
AoSBP: aortic systolic blood pressure
AR: aortic regurgitation
ARi: aortic regurgitation index
CHAI score: composite heart-rate-adjusted haemodynamic-echocardiographic aortic insufficiency score
DD: diastolic delta
HR: heart rate
HR-ARi: heart-rate-adjusted aortic regurgitation index
HRA-DD: heart-rate-adjusted diastolic delta
LVEDP: left ventricular end-diastolic pressure
PVAR: paravalvular aortic regurgitation
TAVI: transcatheter aortic valve implantation
TAVR: transcatheter aortic valve replacement
TOE: transoesophageal echocardiography
Introduction
It has been established that paravalvular aortic regurgitation (PVAR) is an important complication after transcatheter aortic valve implantation (TAVI) and is associated with increased mortality1-4. Echocardiography is presently the modality of choice in the comprehensive periprocedural assessment of post-TAVI aortic regurgitation (AR). It has been employed in core lab evaluations of this complication and can evaluate both severity and mechanism, distinguishing valvular from paravalvular (PV) AR5. However, the quantification of PVAR using echocardiography is challenging, particularly in the intermediate range of severity; indeed, the survival of patients with mild and moderate-severe PVAR was similar in the PARTNER trial1. Moreover, the SAPIEN device (Edwards Lifesciences, Irvine, CA, USA) in the PARTNER IIB trial showed twice the rate of paravalvular AR compared with the PARTNER 1B trial, with the only real change being the different core lab for echocardiographic assessment between the studies. This heterogeneity of assessment has important implications in cross-trial and cross-device comparisons.
Recently, the aortic regurgitation index (ARi) has offered incremental value to angiographic assessment in the risk stratification of PVAR6,7. However, it is known that heart rate (HR) can influence diastolic transcatheter haemodynamics8 and can therefore dramatically alter the ARi, which does not take into account the influence of HR, and has resulted in considerable discrepancies in isolated cases in our large TAVI practice. We hypothesised that a composite heart-rate-adjusted haemodynamic-echocardiographic aortic insufficiency (CHAI) score would improve prognostication of PVAR evaluated by Valve Academic Research Consortium-2 (VARC-2) derived transoesophageal echocardiography (TOE) grading alone9 and also a composite haemodynamic-echocardiographic aortic insufficiency without heart rate adjustment (CAI) score, in line with a recently proposed methodology6.
Methods
PATIENT POPULATION, ASSESSMENT AND PROCEDURE
All patients had severe symptomatic aortic stenosis (AS) and were treated in a single centre with balloon-expandable TAVI (Edwards SAPIEN/SAPIEN XT; Edwards Lifesciences), performed predominantly under fluoroscopic guidance, as has been previously described10. All patients studied had simultaneous transcatheter transaortic haemodynamic pressures measured post TAVI, with a multipurpose catheter placed across the transcatheter valve into the left ventricular cavity and a pigtail catheter placed in the aortic root above the transcatheter valve. If an additional manoeuvre was performed, such as valve-in-valve or post-dilatation, haemodynamic pressures were recorded after that additional intervention.
Patients also had periprocedural TOE imaging for procedural guidance and post-TAVI evaluation of valvular function. TOE was performed using the iE33 xMATRIX Ultrasound System for Echocardiography (Philips Medical Systems Inc., Bothell, WA, USA). Within the confines of available transcatheter haemodynamic data, patients were consecutive and all were followed beyond one year after the index procedure (all patients had at least one year of post-procedural follow-up).
Sizing for TAVI was made at the operator’s discretion, using data from all available imaging modalities at the time of the procedure, with a reliance on traditional cut-offs for annular size by 2D-TOE measurement (D2D-TOE) early in the series, and a later reliance predominantly on cross-sectional measurements by computed tomography or three-dimensional (3D) echocardiography11,12.
POST-TAVI PVAR, THE ARI AND HEART RATE
Post-TAVI PVAR was assessed retrospectively using VARC-2 criteria, which include both semiquantitative and quantitative parameters, with comprehensive periprocedural TOE examinations reviewed retrospectively. This was performed by one of two physician readers experienced in the assessment of TAVI echocardiograms, blinded to the periprocedural TOE report, annular measurements, clinical, angiographic and haemodynamic data, to retain complete objectivity. Reproducibility was excellent: for intra-observer agreement for the assessment of significant PV regurgitation, the kappa statistic was 0.77 (p<0.001), and for inter-observer agreement the kappa was also 0.77 (p<0.001)4. The transcatheter ARi index was calculated according to the following formula: ([DBP-LVEDP]/SBP)×1006. An ARi <25 was regarded as clinically significant6. The heart rate (HR) was derived from the simultaneous electrocardiogram using the R-R interval associated with the haemodynamic waveform studied with stable electrocardiogram and haemodynamics for at least three beats. This was used to generate the heart-rate-adjusted diastolic delta (HR-DD), calculated as (DD/HR*80), where diastolic delta was (aortic diastolic pressure minus left ventricular end-diastolic pressure).
STATISTICAL ANALYSIS
Statistical analyses were made using SPSS software (PASW v18; SPSS Inc., Chicago, IL, USA) and MedCalc v12.7.0 (MedCalc, Ostend, Belgium). Normality of distributions for continuous variables was tested using the Shapiro-Wilk test. The Fisher’s exact test was used for categorical variables compared across independent groups. For normally distributed continuous variables compared across independent groups, an independent samples t-test was employed. For non-normally distributed continuous variables compared across independent groups, a Mann-Whitney U test was used.
Other haemodynamic parameters were also studied for their predictive value for one-year mortality using receiver operator characteristic (ROC) curve analysis. The haemodynamic parameter most predictive of survival was combined with TOE AR grade data to generate an optimal composite (TOE/haemodynamic) heart-rate-adjusted AI (CHAI) score. This was based on the TOE AR grade if there was none/trivial (graded CHAI 0) or severe AR (graded CHAI 3) on TOE, or on a combination of TOE and heart-rate-adjusted transcatheter haemodynamics if there was intermediate AR (mild or moderate) (Figure 1). Intermediate PVAR was graded as not significant if the HR-DD was ≥25 (CHAI score 1) and significant if the HR-DD was <25 (CHAI score 2). A composite AI (CAI) score, recently proposed by the Bonn group, incorporating the ARi without heart rate adjustment has suggested that AR ≥moderate by angiography or echocardiography be regarded as significant and the ARi (<25) be used to stratify mild AR for significance (Figure 1). Other details of statistical analysis performed can be found in Online Appendix 1.
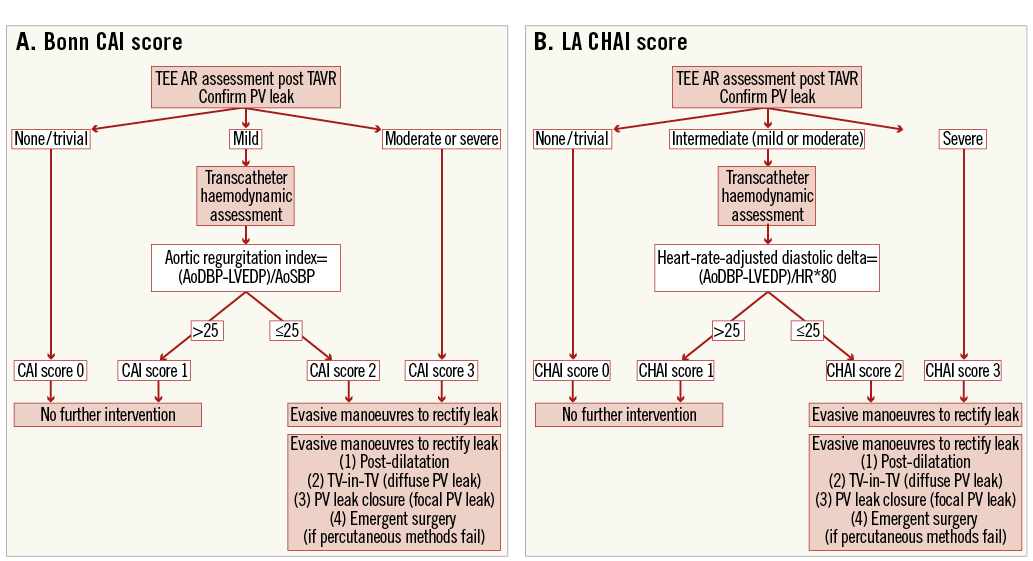
Figure 1. Stratification of intermediate severity aortic regurgitation using the Bonn CAI score (A) and the LA CHAI score (B). There are two principal differences. Firstly, stratification of moderate AR is not performed with the CAI score, whereas the CHAI score does not propose intervention with moderate AR which is not haemodynamically significant. Secondly, the CAI score does not adjust for heart rate, whereas the CHAI score does.
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
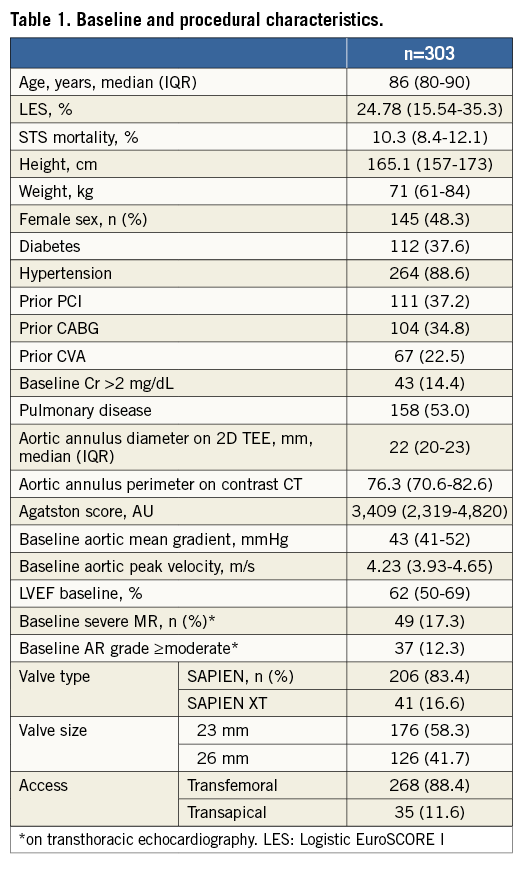
A total of 303 patients were studied. Median age was 86 (interquartile range [IQR], 80-90) and mean aortic valve gradient was 43 mmHg (IQR 41-52). Baseline and procedural data are shown in Table 1.
PROGNOSTICATION OF PVAR BY TOE GRADING OF SEVERITY
By TOE VARC-2 criteria, 145 had no/trivial PVAR (47.9%), 91 had mild PVAR (30.0%), 62 had moderate (20.5%) and five severe PVAR (1.7%). Overall, PVAR by TOE stratified survival poorly (Figure 2). Although there was an excellent prognosis if there was no or trivial PVAR by TOE, there was considerable overlap in outcomes amongst patients in the intermediate range of echocardiographic severity with mild and moderate/severe PVAR having similarly poor outcomes (Figure 2).
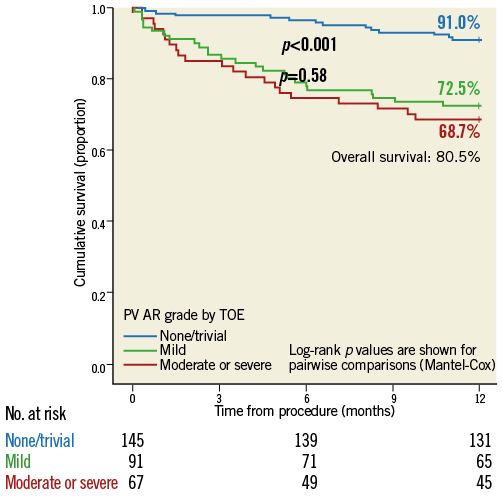
Figure 2. Kaplan-Meier survival curves compared by data available immediately post TAVI in the form of PVAR grade by TOE, graded by VARC-2 criteria. Survival to one-year follow-up is shown.
OPTIMAL DIASTOLIC HAEMODYNAMIC INDICES FOR PROGNOSTICATION
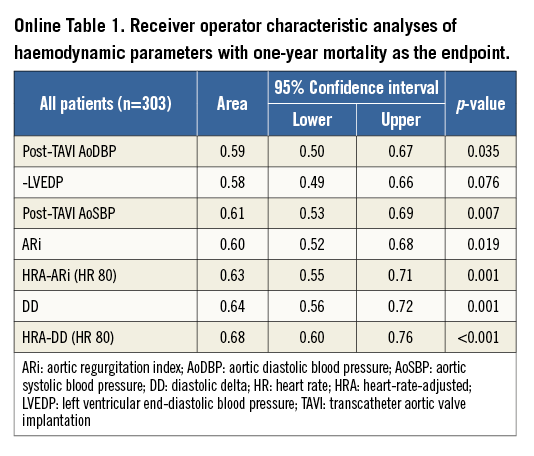
We further studied transcatheter haemodynamic parameters related to survival. A comparison of the individual components of the ARi (AoDBP, LVEDP and AoSBP) showed the “diastolic delta” (DD, the difference between aortic diastolic and LV end-diastolic pressure) to have the greatest predictive value for one-year mortality (Online Table 1). This improved further with simple heart rate adjustment (Diastolic delta/HR*80). Simple heart rate adjustment of the DD dramatically improved the stratification of one-year survival (Online Figure 1). The heart-rate-adjusted diastolic delta (HRA-DD) removed the substantial influence of bradycardia on transcatheter haemodynamics (Figure 3) and was therefore the preferred haemodynamic parameter. The highest sum of sensitivity and specificity for one-year mortality by the HRA-DD occurred at a threshold of ≤24.8. The cut-off of 25 was therefore retained for simplicity. Of note, although the AoSBP is the denominator of the ARi (and hence a lower AoSBP would increase the ARi), lower AoSBP was associated with higher one-year mortality (Online Table 1).
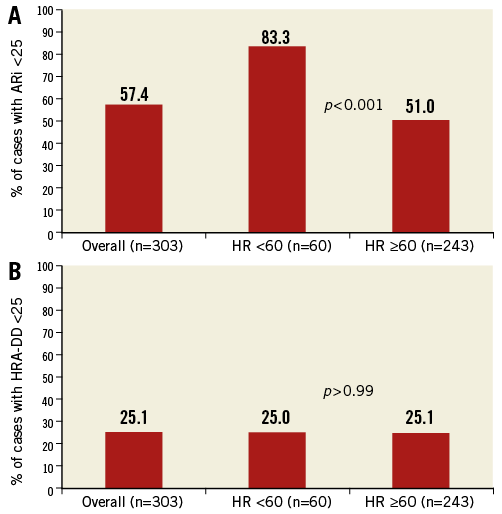
Figure 3. The influence of bradycardia on key transcatheter haemodynamic parameters (ARi and HRA-DD). The ARi is significantly influenced by relative bradycardia (HR <60) whereas the HRA-DD is not. ARi: aortic regurgitation index; HRA-DD: heart-rate-adjusted diastolic delta
A COMPOSITE HAEMODYNAMIC-ECHOCARDIOGRAPHIC AORTIC INSUFFICIENCY ASSESSMENT (CAI)
Composite haemodynamic-echocardiographic assessment using the CAI score, in line with the methodology proposed by the Bonn group, stratified survival somewhat better than TOE alone (Figure 4). However, the haemodynamically non-significant CAI score patients still had a prognosis that was clearly disparate from the group with no/trivial AI and intermediate between the former and the significant CAI score group.
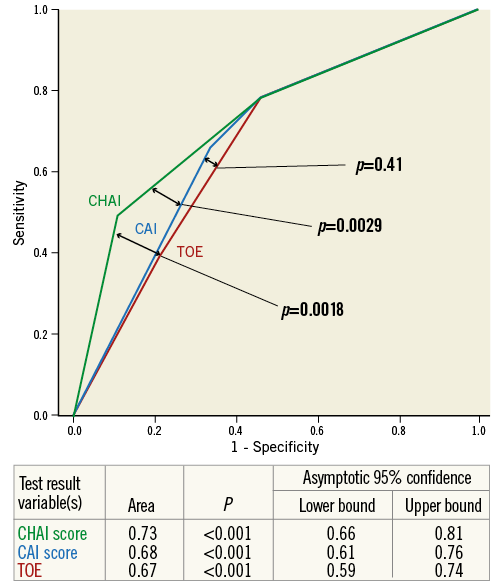
Figure 4. ROC curves comparing the discrimination of one-year mortality by TOE PVAR grade, Bonn CAI score and LA CHAI score.
INCREMENTAL PROGNOSTIC VALUE OF THE CHAI SCORE: SURVIVAL
Since the extremes of PVAR by TOE stratified survival well, transcatheter haemodynamics were not applied for these patients, who retained their TOE grade separation in the composite TOE-haemodynamic grading (zero for none/trivial and three for TOE graded AR ≥3). Given the difficulty in assessing “intermediate” (mild or moderate) and the superimposed outcomes seen in this range (Figure 2), the simple heart rate adjustment of the diastolic delta (Diastolic delta/HR*80) was applied to these patients and those with a value ≥25 were graded 1 in the CHAI score and those with a value <25 were graded 2.
The CHAI score was compared to the CAI score and TOE alone for discrimination of one-year mortality using ROC curve analysis (Figure 4): the composite assessment without heart rate adjustment (Bonn CAI score) was not superior to TOE (Bonn CAI score AUC 0.69, 95% CI: 0.63 to 0.74 vs. TOE AUC 0.67, 95% CI: 0.62 to 0.72, p for difference 0.30). In contrast, the composite assessment with heart rate adjustment (Los Angeles [LA] CHAI score) was superior to both TOE (LA CHAI score AUC 0.73, 95% CI; 0.68 to 0.78 vs. TOE AUC 0.67, 95% CI: 0.62 to 0.72, p for difference 0.002) and the Bonn CAI score (LA CHAI score AUC 0.73, 95% CI: 0.68 to 0.78 vs. Bonn CAI score AUC 0.69, 95% CI: 0.63 to 0.74, p for difference 0.006).
A scrutiny of Kaplan-Meier survival curves based on stratification of PVAR by the respective composite haemodynamic-echocardiographic methodologies demonstrated an improved prognostic stratification of “clinically significant” (score 2 or 3) vs. “not clinically significant” but >trivial (score 1) PVAR with the LA methodology vs. the Bonn methodology, demonstrating the incremental value of heart rate adjustment (Figure 5).
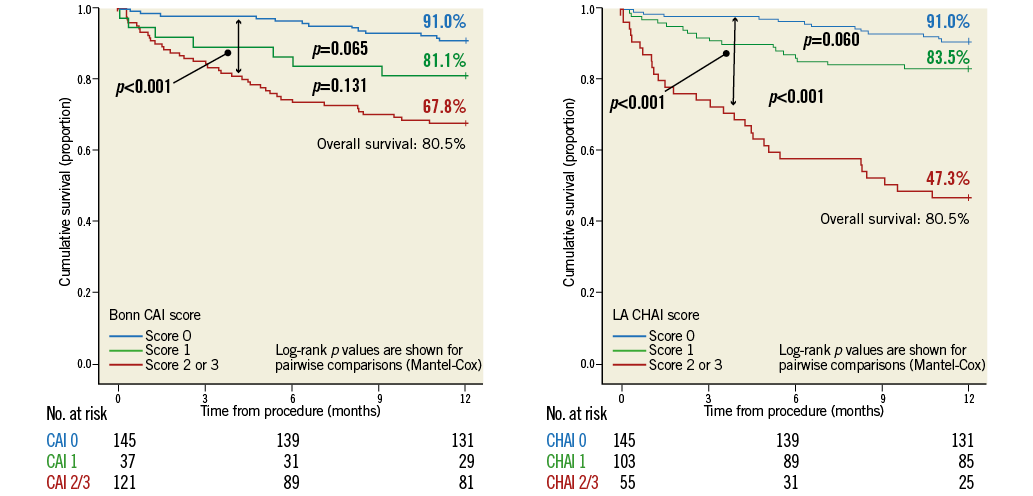
Figure 5. Kaplan-Meier curves stratifying survival by Bonn CAI score (A) and LA CHAI score (B).
THE CHAI SCORE, LEFT VENTRICULAR CHAMBER DIMENSIONS AND NATRIURETIC PEPTIDES
A CHAI score >1 vs. ≤1 stratified the one-month left ventricular end-systolic dimension expressed as a percentage of baseline: this was 106% baseline (IQR 93.8-119.2) vs. 96% baseline (IQR 88-110.2), respectively (p=0.019). Neither AR ≥moderate vs.
Percentage change in serum natriuretic peptide (NPA) levels at one to three months post procedure relative to baseline did not differ significantly in those with AR ≥moderate vs.
COEXISTING CONDITIONS INFLUENCING DIASTOLOGY
Both mitral regurgitation (MR) and left ventricular dysfunction can influence LVEDP and potentially also the DD and CAI/CHAI scores. Baseline severe MR was weakly correlated to baseline LVEDP (p=0.014, r=0.15) but not post-TAVI LVEDP (p=0.46) or significant CHAI score (p=0.63). Lower baseline LVEF was weakly correlated to baseline LVEDP (p=0.010, r=0.15) as well as post-TAVI LVEDP (p=0.043, r=0.12) and significant CHAI score (p=0.037, r=0.12).
MULTIVARIABLE ANALYSIS OF ONE-YEAR MORTALITY AND THE OPTIMAL PROGNOSTICATOR FOR PVAR
In univariate analysis, variables related to one-year mortality to a p<0.1 included age, male sex, baseline creatinine >2 mg/dL, pulmonary disease, STS score, baseline peak velocity, heart rate, LV ejection fraction, PVAR ≥moderate by TOE, CAI score ≥2 and CHAI score ≥2. In the multivariable model without CAI and CHAI scores, PVAR ≥moderate by TOE was not a statistical predictor of one-year mortality (p=0.072), whereas male sex (OR 4.11, 95% CI: 1.93-8.76, p<0.0001), baseline creatinine >2 mg/dl (OR 2.78, 95% CI: 1.29-5.98, p=0.009) and HR (per 10 beats-per-minute increase in HR, OR 1.22, 95% CI: 1.02-1.45, p<0.030) were significant independent predictors. The CAI score was a significant independent risk factor for death when it was added to the model (OR 3.31, 95% CI: 1.60-6.84, p=0.001). In turn, addition of the CHAI score to this model rendered the CAI score non-significant (p=0.12), whereas the CHAI score emerged as the dominant predictor of death at one year (OR 6.5, 95% CI: 3.1-13.8, p<0.001) when the three competing variables assessing PVAR were all included in the model.
Discussion
In this study, we found a substantial incremental prognostic value in the application of a composite heart-rate-adjusted haemodynamic-echocardiographic aortic insufficiency (CHAI) score of paravalvular regurgitation after balloon-expandable TAVI. This builds upon the crucial concept of incorporating transcatheter haemodynamics into the traditional assessment of PVAR6. We found that the CHAI score could also stratify changes in left ventricular cavity dimensions at follow-up, and to some extent changes in serum natriuretic peptides, whereas TOE and a composite score without heart rate adjustment could not. Importantly, this assessment took into account the substantial influence that heart rate has on transcatheter haemodynamics, which has thus far been neglected. A simple illustration of the influence of heart rate on transcatheter haemodynamics can be demonstrated with transvenous pacing where the ARi was <25 or ≥25 in the same patient when the heart rate was lower or higher, respectively (Figure 6).
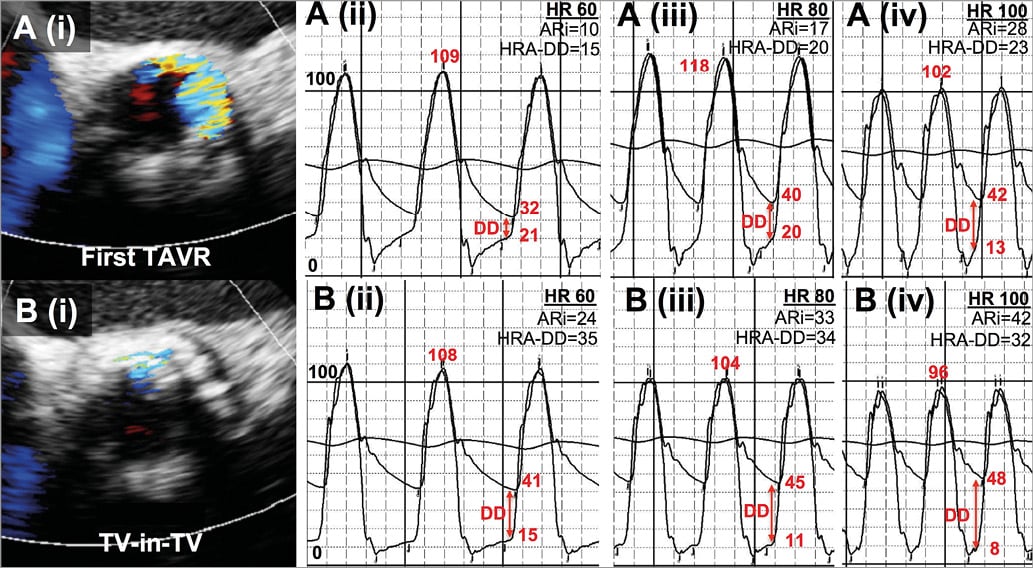
Figure 6. Heart rate and composite echocardiographic-haemodynamic assessment in transcatheter valve-in-valve (TV-in-TV) therapy for severe paravalvular AR due to malpositioning. TOE (i) demonstrated severe AR before the second valve was implanted (A) that resolved to mild immediately post TAVI (B). Heart rate was modified using ventricular pacing. Haemodynamic data (ii)-(iv) are shown pre (A) and post TV-in-TV procedure (B) for heart rates increased with ventricular pacing in the same patient; the haemodynamics vary substantially with heart rate.
ASSESSMENT MODALITIES FOR PARAVALVULAR REGURGITATION AND THE LACK OF A GOLD STANDARD FOR QUANTIFICATION
There remains the lack of a gold standard for the quantification and reliable prognostication of PVAR. Quantification with MRI13 has potential but can be time-consuming and has not been applied routinely. Furthermore, it cannot provide immediate periprocedural information that can be used to guide emergent therapy. Many centres, particularly those employing self-expanding TAVI devices, perform immediate post-procedural angiography, which can be highly observer-dependent, given its reliance on the subjective interpretation of superimposed images4.
The ARi was an important step forward in the prognostic evaluation of AR. However, the frequency of patients with an ARi <25 is very high (57.4% in the present series and 34.2% and 42.6% in two recent series6,7), and often co-exists with no/trivial AR, particularly in the presence of relative bradycardia, which is not infrequently seen after TAVI. We found baseline LVEF to be weakly correlated to LVEDP pre and post TAVI as well as the CHAI score. The CHAI score was a significant independent predictor of mortality, even correcting for baseline LVEF. The presence of LV dysfunction may influence patient tolerance for even mild degrees of AR and hence its influence on the CHAI score may embellish rather than limit the prognostic value of this new composite parameter.
Echocardiography is the mainstay for the identification of paravalvular regurgitation5. However, the quantitative assessment of PVAR by TOE remains challenging and, although grading severe or trivial AR is straightforward, intermediate severity AR has significant inter-observer variability. The difficulties involved are reflected by the fact that, despite a rigorous core lab-based methodology, the PARTNER trial demonstrated equally poor outcomes in patients with PVAR graded as mild vs. those graded as moderate-severe1. Similarly, we observed limited discriminatory value of TOE when the AR was in the intermediate range, such that the Kaplan-Meier curves for mild and moderate PVAR were almost identical (Figure 2). The simplification of assessment by TOE to none/trivial, intermediate and severe, with intermediate further stratified with heart-rate-adjusted haemodynamics is both conceptually appealing and supported by the data presented here.
INTERVENTIONS TO TREAT PARAVALVULAR REGURGITATION AND THE LIMITATION OF RISK
Undoubtedly, PVAR is best avoided through judicious case selection and the application of cross-sectional measures of TAVI sizing11,12,14. Moreover, the advent of new TAVI devices such as the SAPIEN 3 (Edwards Lifesciences) appears to have substantially reduced the frequency of moderate PVAR by echocardiographic assessment but, even with these advances, there remains a substantial percentage of patients with mild PVAR which could still be prognostically significant, particularly with the treatment of younger, lower surgical risk TAVI candidates15. When PVAR occurs, clinicians have several treatment modalities at their disposal during the procedure, including post-dilatation, transcatheter valve-in-valve therapy (TV-in-TV), percutaneous PV leak closure and emergent surgery. Each of these therapies potentially carries additional risk that should be weighed against its potential benefit. Post-dilatation, although often effective in reducing the severity of PVAR, is not only associated with higher rates of clinical stroke16 but is also an important risk factor for the rare but generally fatal complication of annular rupture17. Moreover, transcatheter valve-in-valve therapy (TV-in-TV)18, despite being effective acutely, is associated with higher rates of pacemaker implantation and increased late cardiac mortality18. For percutaneous PV leak closure, although well studied after surgical AVR, there are limited efficacy data in PVAR, where, unlike after SAVR, the leaks are often multifocal. The CHAI score provides an important foundation for stratifying patients who carry the poorest prognosis without further intervention and who thus have the greatest clinical need to justify immediate therapy.
STUDY LIMITATIONS AND FUTURE DIRECTIONS
This was a single-centre retrospective study and its findings require further validation in other prospective series. Although the expert TOE reviewers were blinded to clinical and haemodynamic data, there was no formal core lab evaluation of PVAR by TOE. We did not explore the diastolic:systolic velocity time integral ratio, which is another parameter that may take into account the effects of heart rate on transcatheter haemodynamics and may also be of interest19. Although the CHAI score identifies those patients whose prognosis is favourable and who are thus unlikely to need further intervention, an unfavourable CHAI score does not mean that further manoeuvres to improve AR will necessarily ameliorate outcome. This requires scrutiny in prospective studies. This study employed TOE rather than angiography to stratify none/trivial, intermediate and severe AR, as is the present convention in the USA, and then haemodynamics to stratify further the intermediate AR. Validation of its incremental value in patients whose AR is first stratified using angiography rather than TOE, as is commonplace in Europe, is merited.
Conclusions
Although presently echocardiography is the preferred modality for the assessment of paravalvular regurgitation after TAVI in clinical trials, it is unreliable as a prognostic index when it is in the intermediate range. Diastolic haemodynamic parameters, although valuable, are dramatically influenced by heart rate such that relative bradycardia or tachycardia greatly diminishes their prognostic value. A composite heart-rate-adjusted haemodynamic-echocardiographic aortic insufficiency (CHAI) score offers a considerable improvement in the stratification of risk of paravalvular regurgitation, improving on both echocardiographic grading and non-heart-rate-adjusted haemodynamic parameters.
| Impact on daily practice In this study, prognostication of post-TAVI paravalvular aortic regurgitation (PVAR) in the intermediate range of echocardiographic severity was found to be unreliable and was greatly enhanced by the integration of heart-rate-adjusted transcatheter haemodynamics. Additional manoeuvres to ameliorate paravalvular regurgitation may themselves contribute to adverse events after TAVI and decisions to treat or not to treat acutely may be incorrectly guided by echocardiography or transcatheter haemodynamics without heart-rate adjustment. The presented data support the routine incorporation of heart-rate-adjusted haemodynamics to tailor daily practice in the setting of mild or moderate PVAR. |
Acknowledgements
We acknowledge the valued contribution of Mitch Gheorghiu, Jasminka Stegic, Tracy Salseth, Heera Pokhrel and Jigarkumar Patel to our clinical research programme.
Conflict of interest statement
H. Jilaihawi is a consultant to Edwards Lifesciences, St. Jude Medical and Venus Medtech. O. Sadruddin is an employee of St. Jude Medical. R. Siegel is a consultant to Edwards Lifesciences. R. Makkar has received grant support from Edwards Lifesciences and St. Jude Medical, is a consultant to Abbott Vascular, Cordis, and Medtronic, and holds equity in Entourage Medical. The other authors have no conflicts of interest to declare.
Online Appendix 1. Statistical methods.
ROC curves were generated using post-TAVI one-year mortality as the endpoint (state variable) and VARC-2 TOE AR grade, CAI score and CHAI score as the studied variables. The method of DeLong et al20 was used for direct comparisons of the discriminatory value of one modality to another. Kaplan-Meier curves were also studied for one-year survival stratified according to these respective groups.
A multivariable model for one-year mortality incorporating baseline and periprocedural variables associated with one-year mortality to a significance ≤0.1 was employed using a Forward: LR analysis. This included age, male sex, baseline creatinine >2 mg/dL, pulmonary disease, STS score, baseline peak velocity, heart rate and LV ejection fraction. In order to establish further the dominant prognostic modality assessing PVAR, the three competing parameters PVAR ≥moderate by TOE, CAI score ≥2 and CHAI score ≥2 were progressively added to the model.
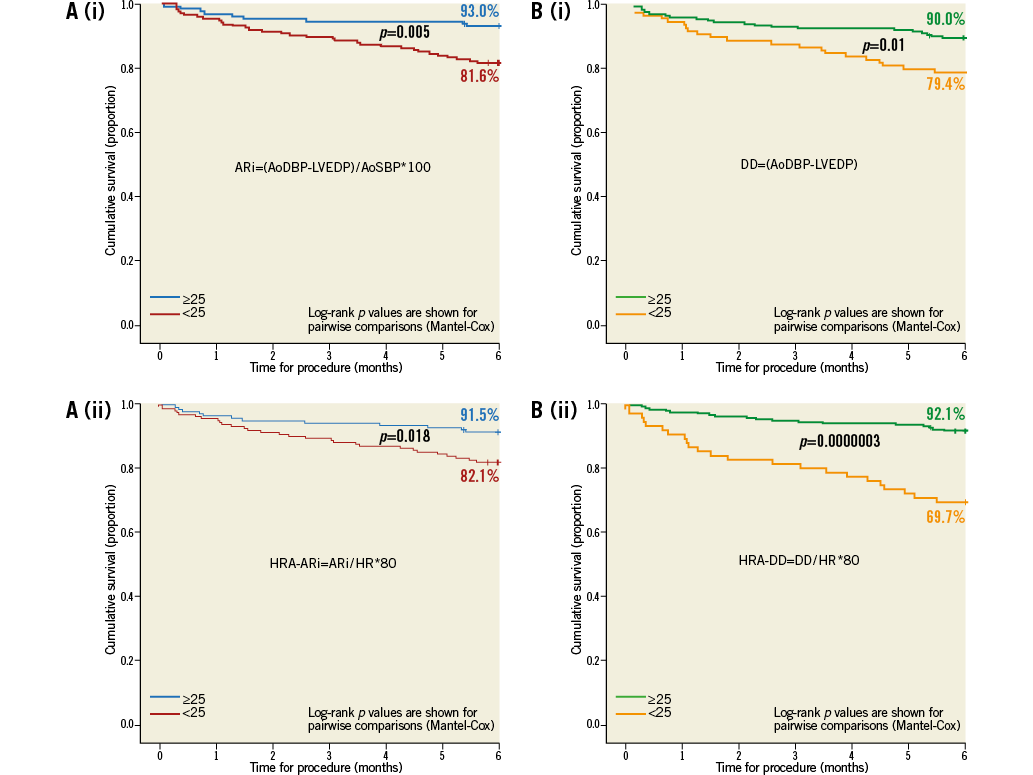
Online Figure 1. Kaplan-Meier survival curves according to immediate post-TAVI haemodynamic data. Kaplan-Meier survival curves are shown for a stratification of transcatheter haemodynamics using the ARi without heart-rate adjustment, panel A(i), and with heart-rate adjustment, panel A(ii), and using the DD without heart-rate adjustment, panel B(i), and with heart-rate adjustment, panel B(ii). Heart-rate adjustment improves the stratification of survival by DD but not ARi.