Abstract
Aims: Transcatheter aortic valve implantation (TAVI) carries the risk of intraprocedural complications that may ultimately require emergent cardiac surgery (ECS). However, few data exist on the incidence, reasons and outcomes of patients needing ECS during TAVI. We analysed data from 2,307 TAVI patients, prospectively enrolled in the multicentre Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry.
Methods and results: Twenty-seven (1.2%) of 2,307 patients required ECS. The rates of ECS were similar for patients undergoing transapical TAVI compared with transfemoral TAVI (1.1% vs. 1.2%). The leading causes for ECS were embolisation/migration of the TAVI valve prosthesis (9/27, 33%) and procedure-related aortic injury (n=7, 26%). Thirty-day mortality of ECS was high (51.9%) and showed cause-specific differences, with 100% mortality in patients with aortic rupture or cardiac tamponade, 0% death in those with acute aortic regurgitation and intermediate risk of death or intermediate mortality in those with aortic injury or valve embolisation/migration.
Conclusions: Rates of ECS during TAVI were low (1.2%). Although ECS was performed without time delay, emergent surgery was associated with a 30-day mortality of 52%. Complications with dramatic acute consequences (annular rupture, aortic injury) had higher mortality than those with less acute deterioration (aortic regurgitation). Prevention of complications requiring ECS during TAVI appears to be of critical importance, focusing on less traumatic, more flexible delivery catheter systems and retrievable valves to reduce the risk of aortic injury and valve embolisation, the two most common causes of ECS.
Introduction
Transcatheter aortic valve implantation (TAVI) is now increasingly used for patients with symptomatic aortic stenosis deemed at high or prohibitive risk for conventional surgery1,2. Like other cardiac procedures, TAVI carries the risk of major complications, some of which may ultimately warrant emergent cardiac surgery (ECS)3. However, data regarding the incidence, reasons and outcomes of ECS during TAVI are scarce. Recent studies have estimated the need for ECS during TAVI to be around 1%4-6.
We analysed data from the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry to understand better the incidence, cause, and outcomes of ECS in patients undergoing TAVI with the balloon-expandable Edwards SAPIEN bioprosthesis (Edwards Lifesciences, Irvine, CA, USA), implanted via the transfemoral (TF) or transapical (TA) approach.
Methods
PATIENT POPULATION
The SOURCE registry was a multicentre, observational, post-approval registry in Europe, designed to monitor the safety and efficacy of the use of the SAPIEN valve in clinical practice7. The registry comprised a total of 2,307 TAVI patients, consisting of 1,038 consecutive patients enrolled at 32 centres between November 2007 and January 2009 (Cohort 1) and 1,269 consecutive patients enrolled at 37 centres between February 2009 and December 2009 (Cohort 2)8. The design of the registry has been previously described in detail8,9. Among the total of 2,307 patients, 35 patients were identified from the database as having undergone cardiac surgery after TAVI (Figure1). In four of them, the local investigators were contacted for data inconsistencies, confirming that these patients actually did not undergo surgery since TAVI was successfully performed. Additionally, three patients had to be excluded as TAVI was not performed due to intraoperative findings of too large anatomy, and patients were referred for elective aortic valve replacement. A single patient was excluded from the analysis due to insufficient data.
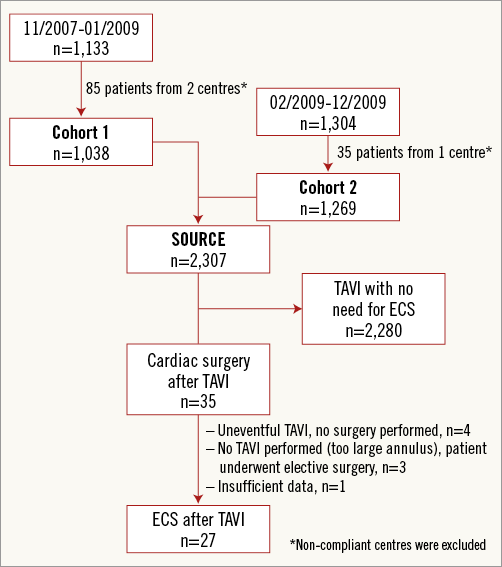
Figure 1. Flow chart of patient inclusion in the SOURCE registry.
PROCEDURES AND DEVICES
In all patients the SAPIEN valve (Edwards Lifesciences) was used. This valve is a balloon-expandable, trileaflet bovine pericardial tissue valve mounted into a balloon-expandable stent as has been previously described in detail7. The valves were available in 23 and 26 mm diameter sizes. A TF or TA approach was used to deliver the valve retrogradely or antegradely. Patients at high risk for conventional surgical aortic valve replacement or those who were considered inoperable were eligible to be treated with this device7. Typically, these patients had a EuroSCORE of >20. Patients being treated by the TF approach were required to have femoral and iliac vessel diameters of >7 mm7.
DEFINITIONS
ECS was defined as any cardiothoracic surgical intervention with cardiopulmonary bypass for acute TAVI complications requiring urgent aortic valve replacement, repair of myocardial or aortic injury, or pericardial drainage performed within the first 24 hours after TAVI4. Surgical procedure(s) needed for vascular access-site complications were not included in this definition. In addition, patients who only needed cardiopulmonary bypass for haemodynamic support during TAVI, but no cardiothoracic surgical intervention, were excluded from this analysis.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±1 standard deviation and categorical variables are presented as percentages. P-values are based on a two-sample t-test for continuous variables and Fisher’s exact test for binary variables. A p-value of <0.05 was considered to denote statistical significance.
Results
The mean age for all patients enrolled in the SOURCE registry was 81.2±6.6 years, and 57% of them were females. A total of 27 out of 2,307 patients (1.2%) required ECS (Table 1). There were no differences in baseline clinical characteristics between patients requiring ECS and those who did not (Table 2). Echocardiographic severity of aortic stenosis was similar, but annular dimensions appeared to be larger in patients requiring ECS. This difference appeared to be accentuated with the use of the smaller 23 mm valve (Table 2).
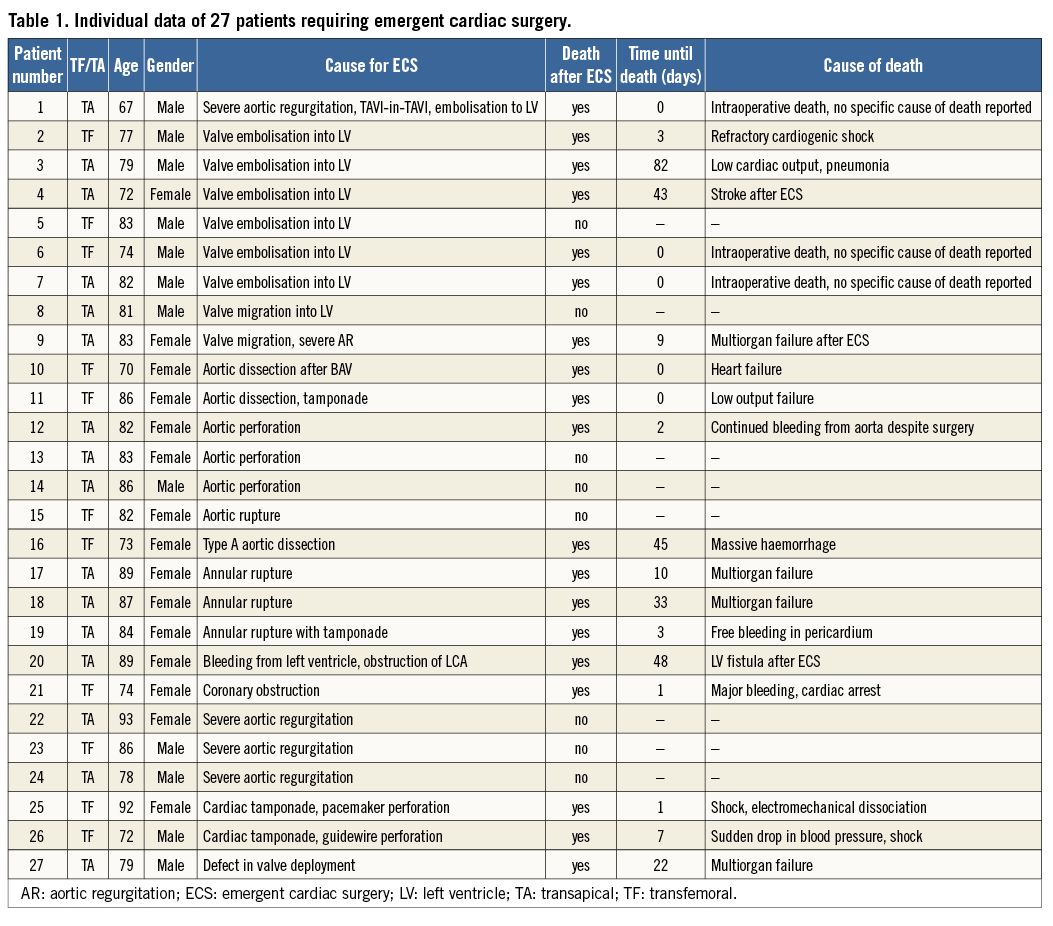
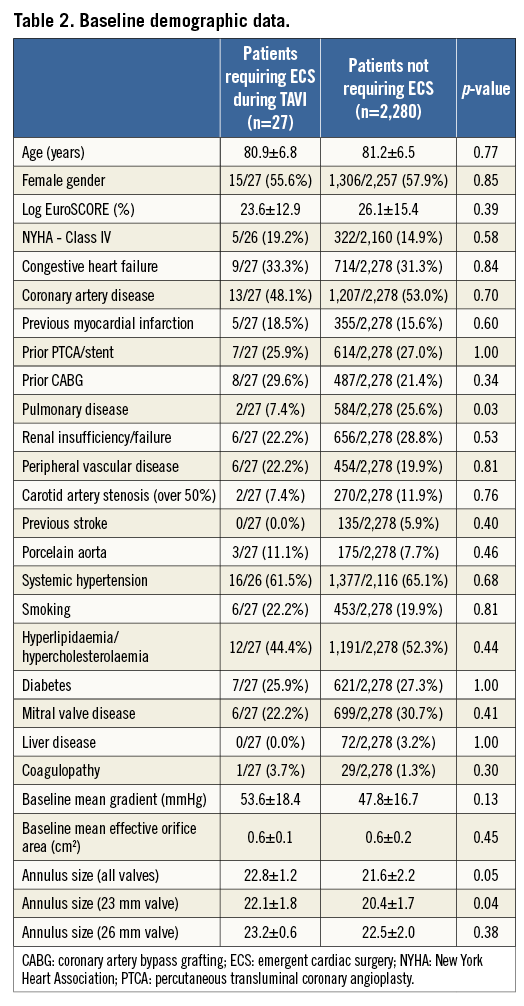
With respect to procedural data, no differences between groups were noted regarding the TAVI access site and valve sizes used: approximately 40% of patients underwent TF TAVI while the majority were treated via the transapical approach (16/27 [59.3%] vs. 1,371/2,280 [60.1%], p=1.00). Similar use of 23 mm (10/26 [38.5%] vs. 945/2,251 [42.0%], p=0.84) and 26 mm (16/26 [61.5%] vs. 1,306/2,251 [58.0%], p=0.84) valve sizes was observed in patients requiring ECS versus those who did not. Procedural times were significantly longer in patients requiring ECS (219.9±129.4 minutes vs. 107.5±48.2 minutes, p<0.001).
CAUSES FOR EMERGENT CARDIAC SURGERY
The leading causes for ECS during TAVI were embolisation/migration of the TAVI valve prosthesis (9/27, 33%) and procedure-related aortic injury including dissection and perforation (n=7, 26%), respectively (Figure 2). Other causes for ECS included rupture of the aortic annulus (n=3, 11%), severe TAVI valve regurgitation (n=3, 11%), cardiac tamponade due to guidewire/pacemaker perforation (n=2, 7%), bleeding from apical access site (n=1, 4%), coronary obstruction (n=1, 4%) and failure in the valve deployment process (n=1, 4%).
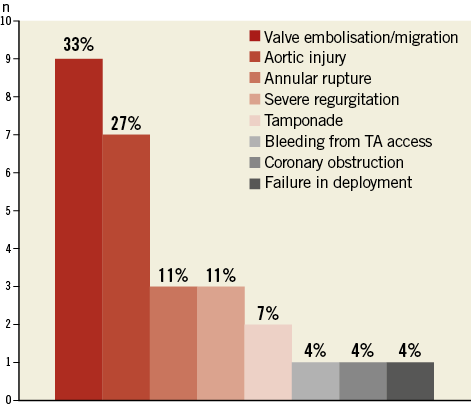
Figure 2. Causes for emergent cardiac surgery during TAVI.
OUTCOMES OF ECS
Immediate mortality according to VARC-2 (death <72 hours)10 occurred in 10 out 27 (37%) patients undergoing ECS. At 30 days, the mortality rate increased to 51.9%, which was sevenfold higher compared to the mortality for patients who did not require ECS following TAVI (7.6%, Table 3). Beyond 30 days, five additional patients died in hospital, resulting in an overall mortality of 70% (19/27 patients) by day 82. Figure 3 depicts the cumulative Kaplan-Meier survival rates of patients who did require ECS versus those who did not.
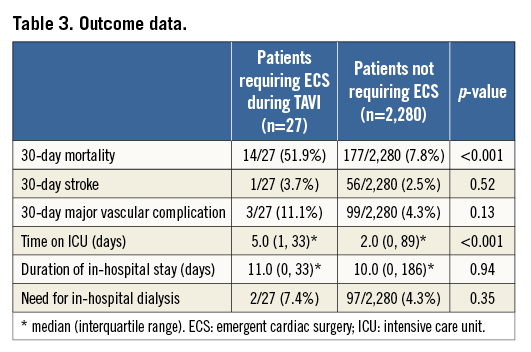
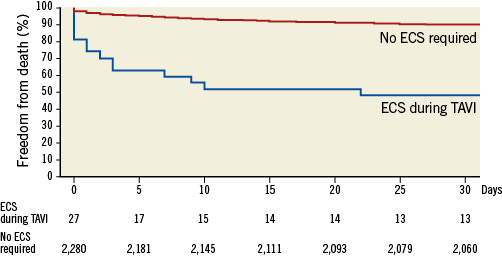
Figure 3. Kaplan-Meier survival curves of patients requiring ECS during TAVI versus those who did not.
Mortality varied broadly among complications requiring ECS. All three patients with annular rupture died despite ECS, as did two patients with cardiac tamponade. In contrast, none of the three patients with severe valve regurgitation died after ECS. Seven out of nine (78%) patients with valve embolisation/migration and 4/7 (57%) with aortic injury died postoperatively.
The most frequent cause of death in ECS patients was low-cardiac-output heart failure/cardiogenic shock – in six out of 19 patients (32%). Four patients (21%) succumbed due to multiorgan failure, four (21%) due to recurrent bleeding and one due to stroke. For the remaining four patients the cause of death was unknown.
COHORT 1 VERSUS COHORT 2
In Cohort 1 (early patients, 2007 to January 2009), 15 (1.4%) out of 1,038 patients required ECS, while in Cohort 2 (February 2009 to December 2009) the incidence was lower (12/1,269, 0.9%, p=0.3). There were some differences in the reasons for ECS between both cohorts. In the earlier Cohort 1, ECS was more often prompted by aortic injury (6/14) as compared to Cohort 2 (2/13). In contrast, in Cohort 2 embolisation of the TAVI valves (7/13) was more present than in Cohort 1 (2/14). Overall mortality after ECS was similar between groups (67% for Cohort 1 vs. 75% for Cohort 2).
TRANSFEMORAL VERSUS TRANSAPICAL TAVI
Rates of ECS were similar for patients undergoing TF TAVI as compared to those undergoing TA TAVI (11/909 [1.2%] vs. 16/1,371 [1.2%], p=0.9). There were some differences among the complications necessitating ECS with the different access routes: in TA TAVI embolisation occurred non-significantly more frequently than in TF TAVI (6/16 vs. 3/11, p=0.69), as did annular rupture (3/16 vs. 0, p=0.28). Mortality rates were similar between patients undergoing TF TAVI versus those undergoing TA TAVI (8/11 [72%] and 11/16 [68%], respectively).
Discussion
Our analysis of the prospective, multicentre SOURCE registry comprising a total of 2,307 patients treated with the balloon-expandable Edwards SAPIEN valve showed that the need for ECS during TAVI was generally low (1.2%), and was similar for TF TAVI and TA TAVI. The leading causes for ECS were embolisation/migration of the TAVI prosthesis as well as aortic injury. The prognosis of patients requiring ECS during TAVI was grave with half of the patients dying within the first 30 days; however, mortality varied among complications requiring ECS.
The low rates of ECS during TAVI in SOURCE reiterate the findings of two recent meta-analyses and an analysis of the German TAVI registry that consistently reported ECS rates as ranging between 1.1% and 1.2%3,4,6. Our analysis further suggests that, similar to decreasing ECS rates for PCI11, this rate may be lower with growing TAVI experience. The rate of ECS was somewhat lower in the more recently treated Cohort 2 (2009, 0.9%) compared with that in the earlier Cohort 1 (2007-2009, 1.4%). The fact that greater experience may lead to a lower ECS rate is further reflected in more recent data from the FRENCH 2 registry comprising 3,195 TAVI patients enrolled between January 2010 and October 2011 which reported a very low ECS rate of only 0.4%5. Similarly, in the recent European ADVANCE study12 using the Medtronic CoreValve prosthesis in >1,000 patients, only a single (0.1%) patient required ECS, suggesting it is likely that the requirement for ECS in recent times may be even lower than that observed in the SOURCE registry.
At present, preoperative identification of patients at increased risk for intraprocedural complications requiring ECS is difficult. Our study demonstrated no differences in baseline characteristics between patients who needed versus those who did not require ECS during TAVI. This is consistent with the findings of a recent single-centre study13. Similar difficulties in predicting emergent reoperation (for bleeding) using baseline preoperative information have been reported even in a very large cohort of patients undergoing cardiac surgery (c-index of the model 0.69 suggesting very modest discriminatory ability)14. This is not surprising given that, while complications of a procedure are perhaps likely to be related to, and could be reasonably predicted based on baseline (and/or preoperative) features, the treatment of a given complication with ECS involves complex physician judgement, tailored to individual patient and other intraoperative factors that are often not captured in observational registries and thus difficult to predict using only baseline characteristics.
The leading causes for ECS in our study were embolisation/migration of the TAVI valve and aortic injury, which accounted for more than half of all ECS during TAVI. Interestingly, annular dimensions were larger in patients requiring ECS after TAVI, particularly with the use of the smaller 23 mm valve. It is thus somewhat tempting to speculate that incomplete anchoring of the TAVI valve in the larger annulus may contribute to the high rate of valve embolisation/migration. Other causes for ECS included annular rupture, severe regurgitation, pericardial tamponade, and coronary obstruction. This is in line with the findings of a previous meta-analysis that also indicated valve embolisation/migration to be the leading cause for ECS4. In a recent analysis of the German TAVI registry, aortic injury comprising aortic perforation/dissection as well as annular rupture was found to be the most frequent cause for ECS, occurring in eight out of 20 (40%) patients6. In the German TAVI registry in which the Medtronic CoreValve device was predominantly used15, this occurred in six of the eight patients with the use of the Edwards SAPIEN prosthesis. In the present analysis of the SOURCE study which included patients treated with the Edwards prosthesis exclusively, the aggregate of aortic injury and annular rupture prompted ECS in 10 out of 27 (37%) patients and was thus comparable to the findings of the German TAVI registry6.
The present analysis suggests some differences in mortality in relation to the causes of ECS. Annular rupture occurred in three of the 2,307 patients (0.1%), and all of them died despite ECS. Annular rupture is among the most dreaded TAVI complications resulting in dramatic acute deterioration of the patient and it is thus somewhat intuitive that ECS for this complication was associated with high postoperative mortality16. In fact, similar high mortality of ECS for annular rupture has been reported in other studies6,13,17. In the series of Pasic et al17, annular rupture occurred in six out of 618 patients (1%) undergoing TA TAVI using the balloon-expandable valve, and three out of five (60%) patients who underwent ECS for this complication died postoperatively. Seiffert et al13 reported annular rupture in two of 458 (0.4%) patients and one of them died following ECS. In the German TAVI registry, annular rupture prompted ECS in three out of the total of 1,975 (0.2%) patients and two (66%) of these patients died postoperatively6. In contrast to annular rupture, severe aortic regurgitation (AR) during TAVI is acutely well tolerated, but negatively affects short to midterm survival18,19. Thus, ECS may be performed less emergently than ECS for annular rupture. In both the present analysis as well as in the German TAVI registry6, severe AR during TAVI prompted ECS in three patients. In the present analysis, all three patients survived, which is in line with the German TAVI registry which reported a survival rate of two out of three patients6. Conflicting data, however, exist about valve embolisation/migration to the left ventricle, which accounted for 1/3 of patients requiring ECS in the present analysis and was thus the single most frequent cause for ECS. Mortality despite ECS approached almost 80%. In the German TAVI registry, prosthesis embolisation was observed with similar frequency (25%), but only two out of five (40%) patients died after ECS for this specific indication.
In contrast to previous analyses reporting higher rates of ECS with TA TAVI3,4,6, we found similar ECS rates for TF TAVI and TA TAVI. We noted some, albeit not statistically significant, differences among the reasons for ECS between the two approaches. In TA TAVI the leading cause for ECS was embolisation/migration of the valve prosthesis, which was twice as frequent compared to TF TAVI. This was somewhat unexpected since TA TAVI is usually considered to allow more precise valve implantation than the TF approach due to the shorter distance from access to target20. Therefore, prosthesis embolisation should be conceptually less frequent than with TF TAVI. One may speculate that impaired imaging, when performing TA TAVI in the operating room using C-arm fluoroscopy, may offset some of the advantage of better device handling via this approach.
Our findings may have some implications. First, given the dismal outcomes of ECS during TAVI, all efforts must be directed at preventing any complications that may warrant ECS. Development of more stable, more easily deployable, retrievable and repositionable TAVI prostheses may further reduce the need for ECS during TAVI and its inherent mortality. Additionally, improved preprocedural imaging by three-dimensional echocardiography, CT or MRI may improve appropriate positioning and sizing. Smaller size and improved flexibility of catheter devices used for TAVI may decrease the risk of aortic injury necessitating ECS. Finally, whether these data provide some guidance for identifying not only a subset of the already high-risk TAVI patients who are likely to benefit from ECS (those with aortic regurgitation), but also the futility of such a procedure in others (those with overt annular rupture) remains to be evaluated in the future.
Study limitations
Our study should be viewed in the light of its strengths and limitations. Unlike previous publications that utilised meta-analyses of published data3,4, our study provides information on patient-level data in a relatively large and consecutive cohort of patients that were carefully collected. Thus, we were able to assess the effects of baseline patient features on the need for ECS and its outcomes. We were also able to provide information on the cause of mortality for specific indications for ECS. However, this ad hoc analysis has some limitations. Given the observational and self-reporting study design of the SOURCE registry, caution needs to be exerted when implying causation. The small number of patients requiring ECS should prevent overinterpretation of our data. Rather, our study should be considered hypothesis-generating, stimulating future carefully designed investigations addressing this issue and confirming its findings. Our findings may not be applicable to self-expandable valves.
Conclusions
In this prospective, multicentre registry of TAVI using the balloon-expandable Edwards SAPIEN valve, rates of ECS during TAVI were low with the most common cause being embolisation/migration of the prosthesis. ECS was associated with a high 30-day mortality of 52%, showing some differences with respect to the specific TAVI complications prompting ECS. Thus, prevention of complications requiring ECS during TAVI appears to be of critical importance. Future TAVI developments should be directed towards development of less traumatic, more flexible delivery catheter systems and retrievable valves to reduce the risk of aortic injury and valve embolisation.
Funding
The SOURCE registry was funded by Edwards Lifesciences. There was no additional funding for this analysis.
Conflict of interest statement
H. Eggebrecht, P. Kahlert, G. Schymik, T. Lefèvre, R. Lange, O. Wendler and M. Thomas are clinical proctors for Medtronic and/or Edwards Lifesciences and have received honoraria payments. L. Mandinov is an employee of Edwards Lifesciences. The other authors have no conflicts of interest to declare in relation to this article.

