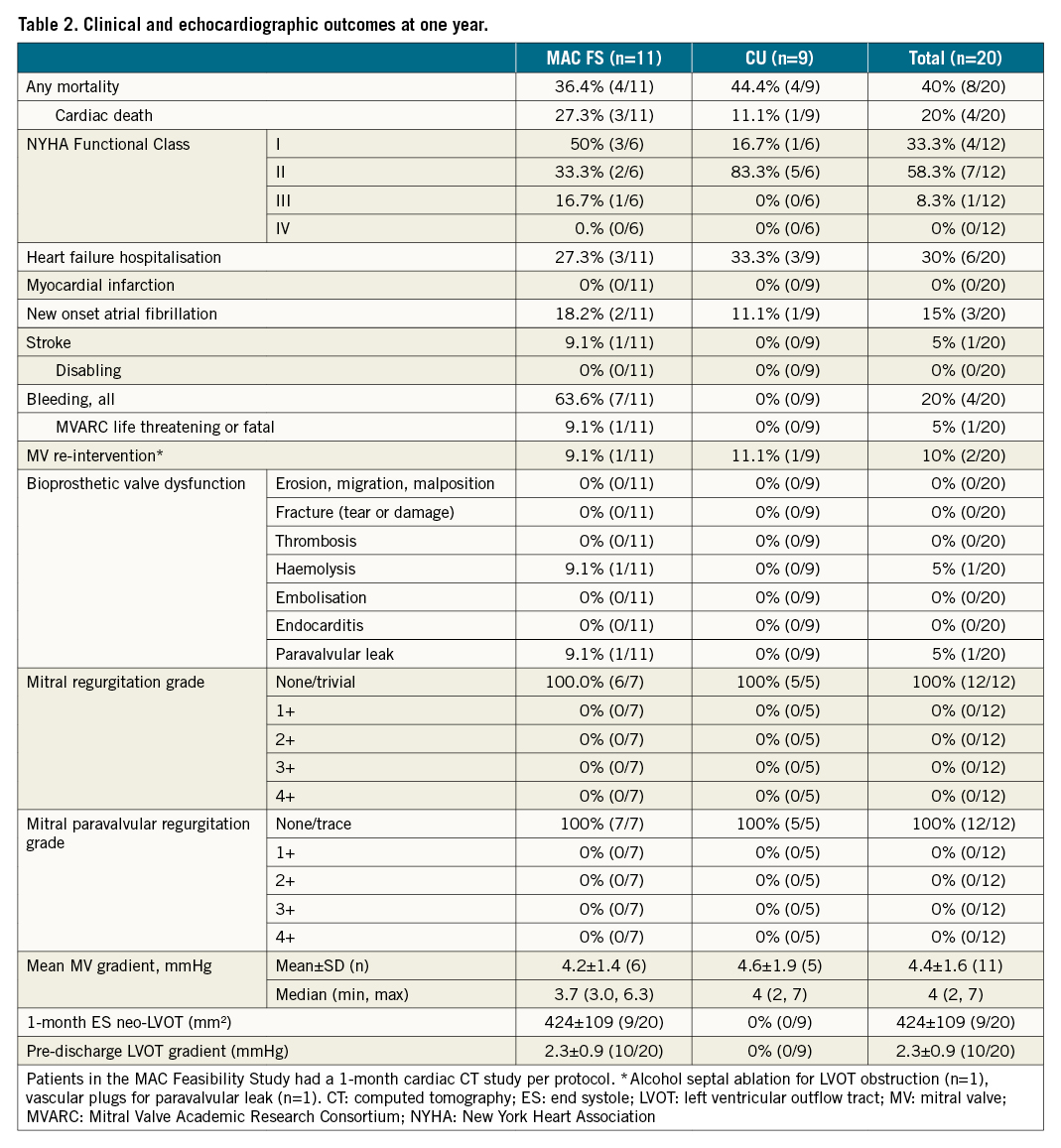
Abstract
Background: Treatment of mitral regurgitation (MR) associated with severe mitral annular calcification (MAC) is challenging due to the high risk of fatal atrioventricular groove disruption and significant paravalvular leak.
Aims: The aim of this study was to evaluate the outcomes of transcatheter mitral valve replacement (TMVR) with the Tendyne valve (Abbott Structural) in patients with MR and MAC.
Methods: Twenty patients (mean age 78 years; 11 women) who were treated with the Tendyne valve, either compassionate use (CU; closed) or as part of The Feasibility Study of Tendyne in MAC (NCT03539458), had reported outcomes in a median follow-up duration of 368 days.
Results: In all patients, a valve was implanted with no procedural mortality and successful hospital discharge. Two embolic events occurred, including one with mesenteric ischaemia and one non-disabling stroke. At 30 days and one year, all-cause mortality occurred in one (5%) and eight patients (40%), respectively. At one year, six patients had been hospitalised for heart failure (30%). There was no prosthetic dysfunction, and MR remained absent in all patients at one year. Clinical improvement, measured by New York Heart Association Functional Class, occurred in 11 of 12 patients who were alive at one year. Among seven survivors with Kansas City Cardiomyopathy Questionnaire (KCCQ) data, mean increase in KCCQ score was 29.9±26.3 at one year with improvement of ≥10 points in five (71.4%) patients.
Conclusions: In patients with MR and severe MAC, TMVR with the Tendyne valve was associated with encouraging acute outcomes, midterm durability, and clinical improvement. Dedicated TMVR therapy may have a future role in these anatomically challenging, high-risk patients.
Introduction
Mitral annular calcification (MAC) results from calcific degeneration or disruption of the native mitral apparatus and, in severe cases, it can be associated with invasion into the myocardium. MAC leads to restricted leaflet motion, annular rigidity, stenosis, and mitral regurgitation (MR). For patients with symptomatic mitral disease and severe MAC, surgery with traditional mitral valve replacement presents a significant challenge due to the risk of atrioventricular disruption, which is often fatal123. Transcatheter edge-to-edge repair is often not feasible due to leaflet restrictions and pre-existing transvalvular gradients. Moreover, there is no transcatheter therapy currently approved for the treatment of symptomatic mitral valve disease in patients with severe MAC, representing an unmet clinical need3.
Transcatheter techniques for the treatment of patients with MAC have been evaluated using a balloon-expandable or mechanically expanding aortic prosthesis in the mitral position (e.g., SAPIEN [Edwards Lifesciences] and Lotus [Boston Scientific])45. More recently, implantation of a prosthesis specifically designed for the mitral valve, the Tendyne system (Abbott Structural), has shown encouraging results in patients with severe MAC, with complete elimination of MR and a favourable safety profile (i.e., no procedural mortality)6. In October 2018, the single-arm, multicentre Tendyne MAC Feasibility Study began enrolling patients with symptomatic severe MR and MAC who were at prohibitive risk for surgical MR correction. The purpose of this study was to evaluate the performance of the Tendyne system to support the expansion of the therapy and to guide the development of further investigations. The present analysis examines the entire present experience of Tendyne for patients with severe MR and MAC, with an aggregation of one-year outcomes from the Tendyne MAC Feasibility Study (FS) (NCT03539458) (n=11) and from patients implanted under compassionate use (CU); n=9. The Tendyne MAC FS closed enrolment on 30 October 2019.
Methods
Study population
The study population comprises two cohorts: CU and the MAC Feasibility Study (NCT03539458). All patients provided informed consent to undergo the procedure under CU or for participation in the MAC FS study, with all procedures approved by the local institutional review boards and conducted in accordance with the Declaration of Helsinski. Each patient was evaluated as a candidate for a transcatheter mitral valve replacement (TMVR) procedure by a complete Heart Team consisting of interventional cardiologists and cardiac surgeons. The Heart Team evaluated the medical history, vitals, laboratory data, transthoracic echocardiography, transoesophageal echocardiography and electrocardiogram (ECG)-gated contrast-enhanced cardiac computed tomography (CT) of each patient7. All medical imaging measurements used to determine anatomical suitability and eligibility criteria were evaluated by a core laboratory. All patients were anatomically screened by the sponsor to assess valve sizing. Device simulation was performed using CT analysis to determine left ventricular outflow tract (LVOT) area and the clearance between the septum and anterior strut of the prosthesis. Anatomically suitable patients were reviewed with site investigators for preprocedural planning and recommendations for concomitant procedures (e.g., balloon valvuloplasty).
Nine patients with severe MAC were treated under CU at five hospitals across the USA and Europe. All CU patients received approval from their respective national regulatory agencies (Food and Drug Administration in the United States, Federal Institute for Drugs and Medical Devices in Germany, or L’Agence nationale de sécurité du médicament et des produits de santé in France). CU patients generally met study criteria for The Expanded Clinical Study of the Tendyne Mitral Valve System (NCT02321514), with the exception of the severe MAC or severe mitral stenosis exclusion.
Eleven patients were recruited for the Tendyne MAC FS from five hospitals in the United States; two of these hospitals also had CU experience with Tendyne TMVR for severe MAC. Key study enrolment criteria for the Tendyne MAC FS were: 1) symptoms of heart failure (i.e., New York Heart Association [NYHA] Functional Class ≥II); 2) severe MR; 3) severe MAC; 4) lack of suitability for surgical mitral valve replacement; and 5) expected benefit from reduction in MR, as determined by a local Heart Team evaluation. Key exclusion criteria were: 1) severe left ventricular dysfunction (ejection fraction <30% or end-diastolic diameter >70 mm); 2) severe tricuspid regurgitation or right ventricular dysfunction; 3) haemodynamic instability; 4) forced expiratory volume in 1 second (FEV1) <1 L or <50% predicted; and 5) life expectancy <1 year. A full description of the MAC FS eligibility criteria is available publicly (https://clinicaltrials.gov/ct2/show/NCT03539458).
An independent echocardiography core laboratory (Beth Israel Deaconess) confirmed subject eligibility. Similarly, a computed tomography core laboratory (St. Paul’s Hospital, British Columbia, Canada) conducted anatomic screening for all subjects. Patients treated under CU had follow-up imaging and outcomes reported by site to the sponsor. Tendyne MAC FS sites submitted follow-up imaging to the core labs and adverse events were adjudicated by an independent clinical events committee (CEC) (Figure 1).
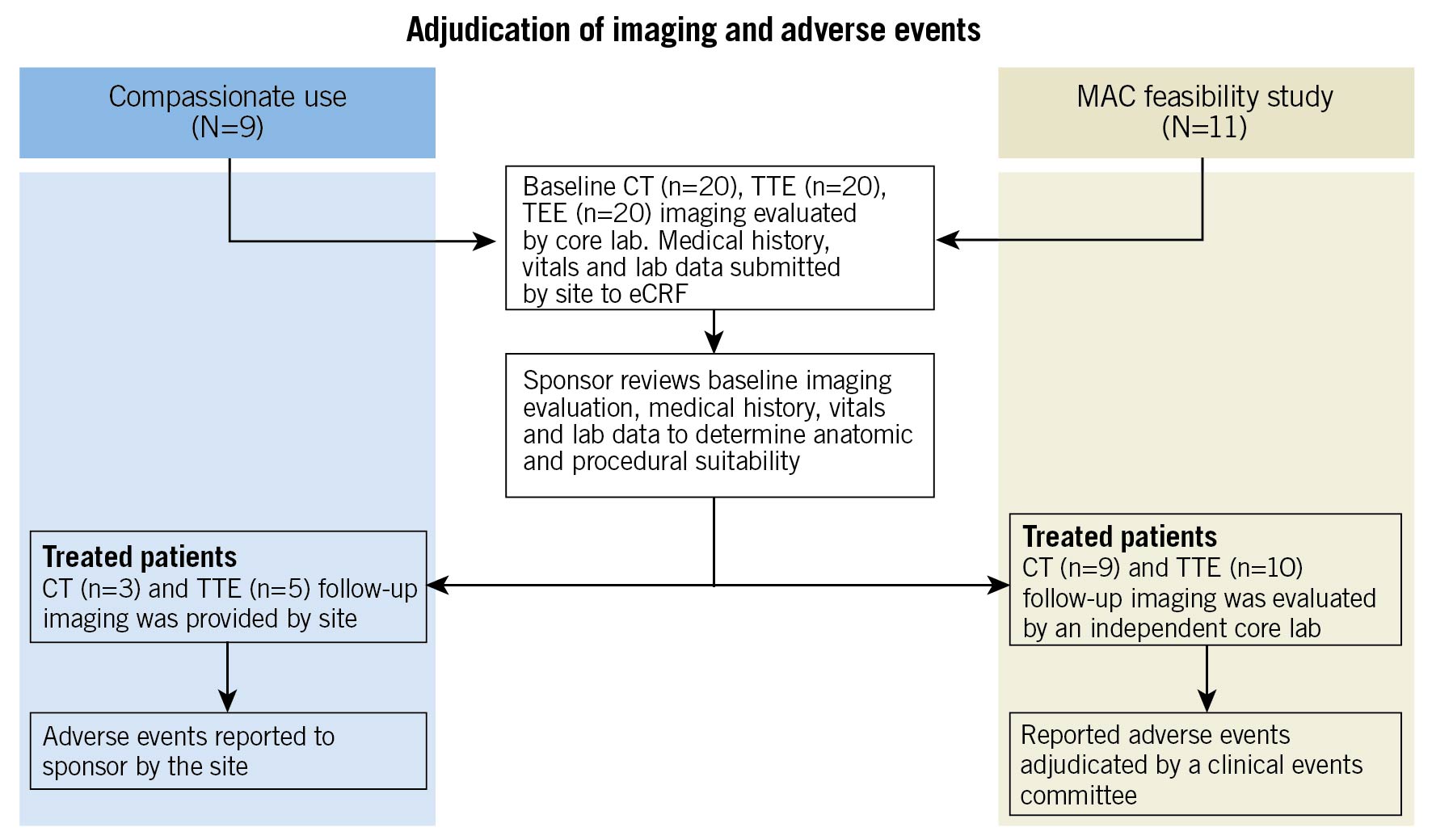
Figure 1. Adjudication of imaging and adverse events. CT: computed tomography; eCRF: electronic case report form; MAC: mitral annular calcification; TEE: transoesophageal echocardiogram; TTE: transthoracic echocardiogram
Transcatheter mitral valve prosthesis and implantation
The Tendyne transcatheter mitral valve system comprises a bioprosthetic valve, tether, epicardial pad, and delivery system that has been described previously6. The prosthesis is deployed transapically via a left lateral thoracotomy, and without the need for cardiopulmonary bypass, rapid ventricular pacing, or haemodynamic support. To ensure the successful deployment of the prosthesis in severe MAC, predilatation of the mitral valve apparatus with a balloon was performed when there was concern regarding optimising expansion of the prosthesis due to calcific spicules or restricted mitral leaflets. Unless medically contraindicated, anticoagulation with warfarin was administered to all patients for at least six months following the Tendyne procedure.
Definitions and data analysis
CT imaging was used to characterise and quantify MAC, categorising it as severe when it posed significant surgical risk, with a minimum total MAC volume of 750 mm3 or when MAC invaded the myocardium. CT images were analysed using the Mimics Innovation Suite (Materialise). For each specific subject, a 3D mask model of the MAC was created using a lower Hounsfield unit threshold value that excluded the blood volume contrast. The MAC volume was obtained from the MAC mask properties. Imaging was also used to assess the distribution of MAC to accommodate valve seating, and the potential impact of the calcified annulus on the inner frame (Figure 2, Figure 3). The distribution of MAC volumes is shown in Supplementary Figure 1. Severe MR was defined using standard American Society of Echocardiography criteria89. Device and technical success were defined using Mitral Valve Academic Research Consortium (MVARC) criteria10. Evidence of MR, device dysfunction (stenosis, degeneration, fracture), malposition (embolisation or migration), and left ventricular outflow tract (LVOT) obstruction were based on two-dimensional and Doppler echocardiography. The Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) for replacement was calculated to determine surgical risk.
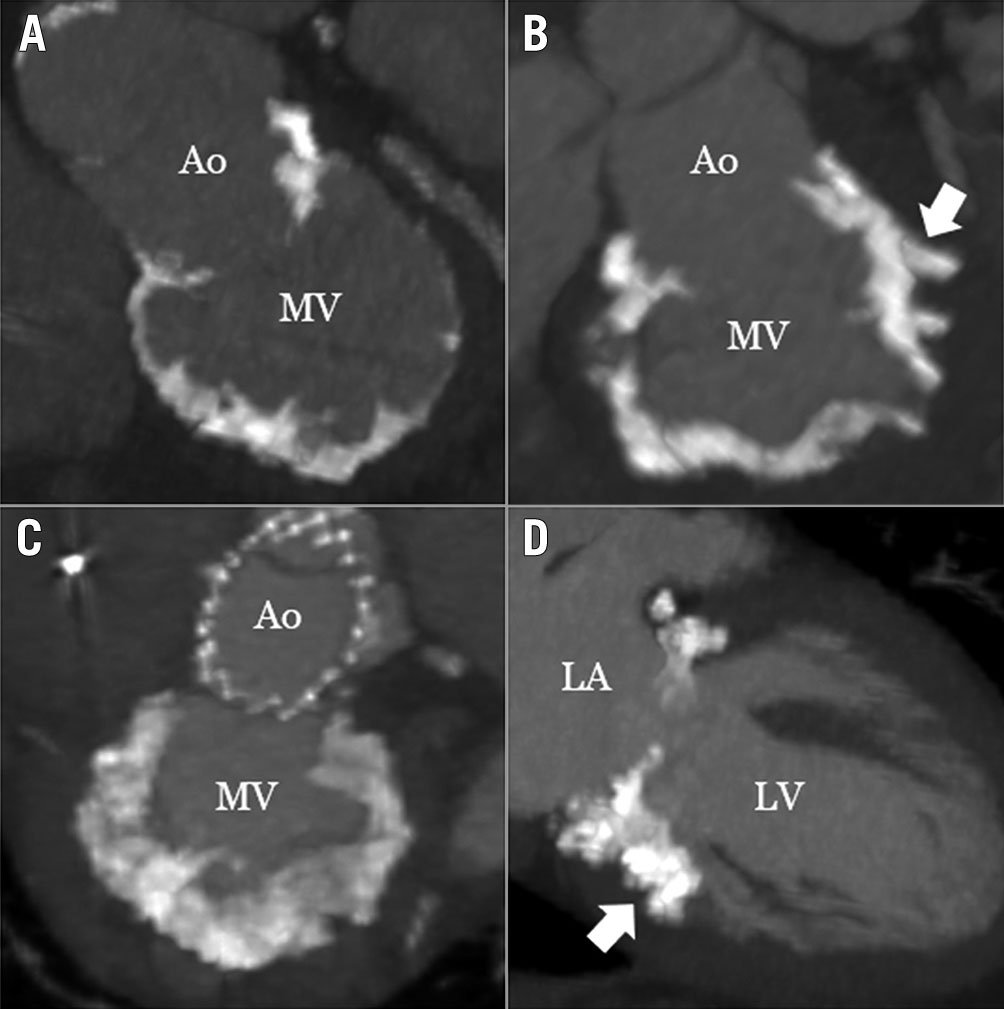
Figure 2. Representative patterns of severe MAC in patients treated with the Tendyne. ECG-gated, contrast-enhanced cardiac computed tomography images from patients treated in this study. Panels A, B, and C are from unique patients; panels C and D are from the same patient. Spicules invading the myocardium were evident in several patients (B and D, arrows). Ao: aorta; ECG: electrocardiogram; LA: left atrium, LV: left atrium; MAC: mitral annular calcification; MV: mitral valve
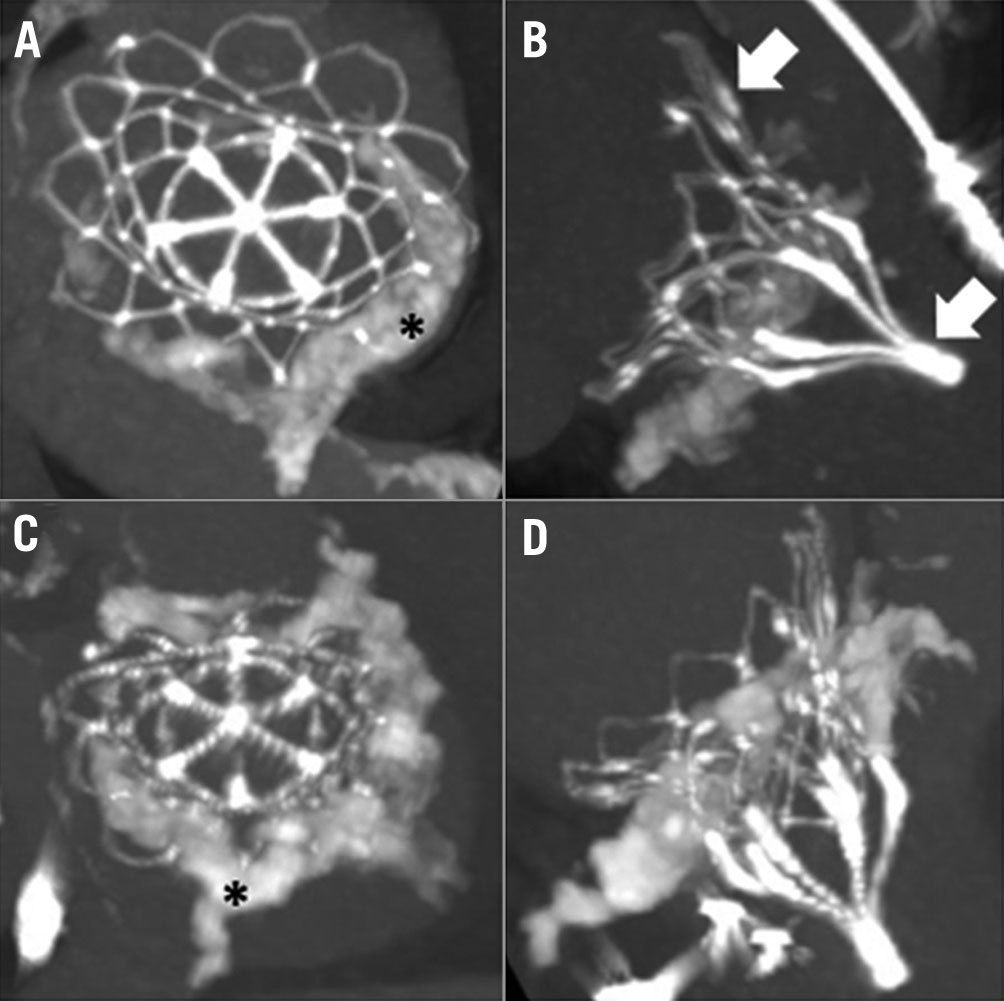
Figure 3. Post-implantation images of patients with severe MAC treated with the Tendyne. ECG-gated, contrast-enhanced cardiac computed tomography images from two representative patients 30 days after implantation. (A and B) and (C and D) correspond to the same patient. The asterisk indicates severe mitral annular calcification. Arrows indicate the prosthesis. ECG: electrocardiogram; MAC: mitral annular calcification
Clinical follow-up was performed at 30 days and one year. All analyses were performed for each individual cohort and as a total study population (n=20). All patients were evaluated for the occurrence of adverse clinical events (i.e., death, stroke, transient ischaemic attack, bleeding, myocardial infarction, endocarditis and haemolysis), need for re-intervention (transcatheter or surgical), and recurrent hospitalisation. Normally distributed continuous variables are presented as means±SD; continuous variables not normally distributed (Shapiro-Wilk test, p<0.05) are presented as median (interquartile range [IQR]).
Results
Study patients
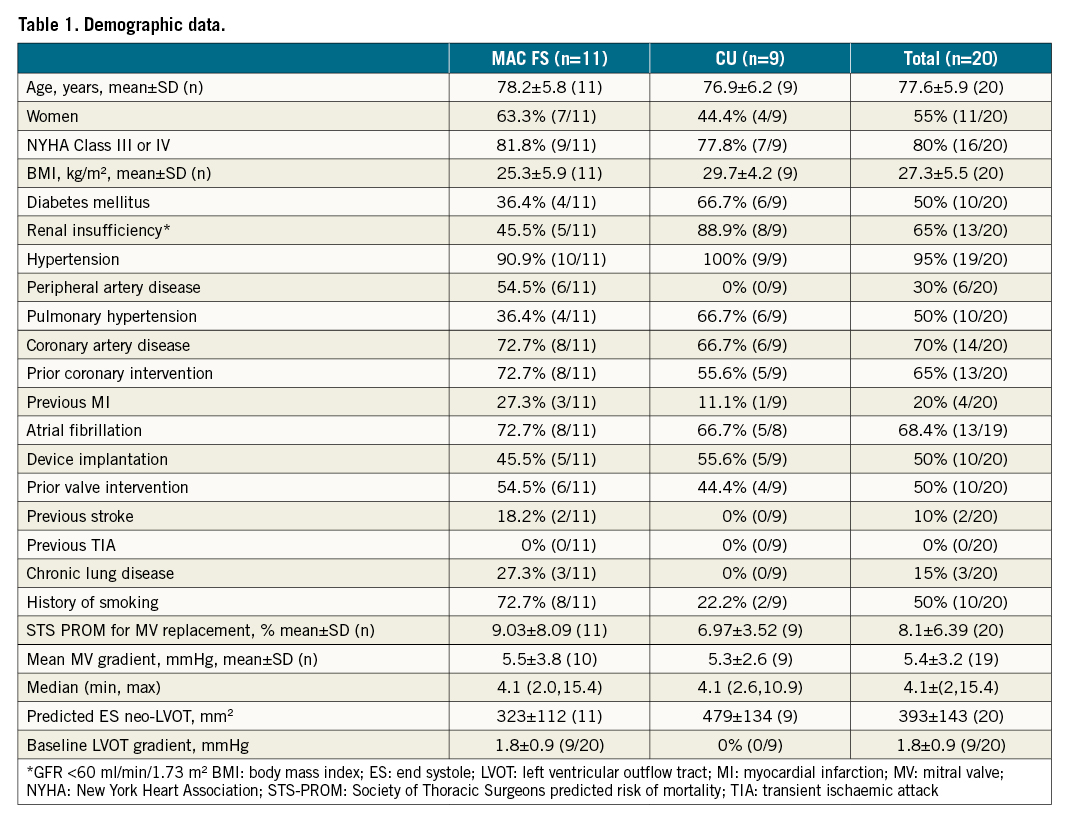
Clinical and demographic characteristics are summarised in Table 1. Patients were elderly (mean age 78±6 years; 11 women) and severely symptomatic, with multiple comorbidities that contributed to a mean STS-PROM of 8.1±6.4%. Of the 20 patients, 16 had preserved left ventricular function (i.e., LVEF ≥50%); the mean LVEF was 58±9%. While all patients had severe MR, 7 out of 19 (31%) had an elevated baseline mean mitral gradient ≥5 mmHg, demonstrating stenosis. In one patient there was severe stenosis in addition to regurgitation. Among the 15 patients with a calculated mitral valve area, all were <4 cm2. Those who were treated as part of the MAC FS had slightly higher STS-PROM scores compared to CU cases (9.0±8.1% vs 7.0±3.5%), attributable to higher rates of lung disease, coronary artery disease and prior stroke.
Overall haemodynamic performance was improved, MR was eliminated in all patients and mean mitral valve gradient was reduced to 4.4±111. Mitral regurgitation was eliminated to trace or less in all patients, with no observed paravalvular leaks (Table 2).
The range of CT-derived MAC volumes was 799 to 18,041 mm3, with an average of 4,273±4,053 mm3 (Supplementary Figure 1). Circumferential MAC (i.e., >270 degrees) was present in 13 patients, all of whom had calcium protruding from the mitral annulus directed either into the myocardium or towards the left ventricular cavity.
Procedural and in-hospital outcomes
The Tendyne device was implanted in all patients with complete elimination of MR. There was no procedural mortality, no device embolisation, and no significant stenosis of the bioprosthesis (Table 3). No cases required cardiopulmonary bypass or peripheral extracorporeal membrane oxygen (ECMO). An intra-aortic balloon pump was used in one patient (5%). Pre-implantation balloon valvuloplasty was performed in 11 (55%) cases. Fifteen patients received a low-profile prosthesis to accommodate small left heart anatomy, and to minimise the risk of LVOT obstruction. One patient experienced LVOT obstruction intraprocedurally, which was successfully treated with alcohol septal ablation6. Therefore, technical success, according to MVARC criteria, was 95% (19 out of 20 patients). The median hospital stay was seven days, including a median ICU stay of 2.6 days. Thirteen patients (65%) were discharged directly home, with the remainder entering skilled nursing or transitional care facilities for continued postoperative rehabilitation.
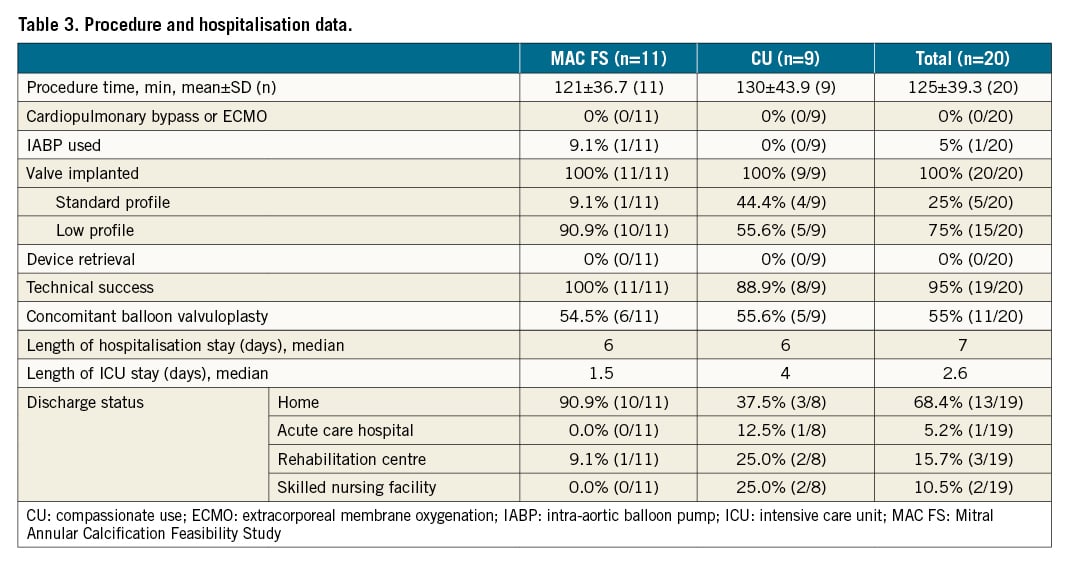
Clinical outcomes
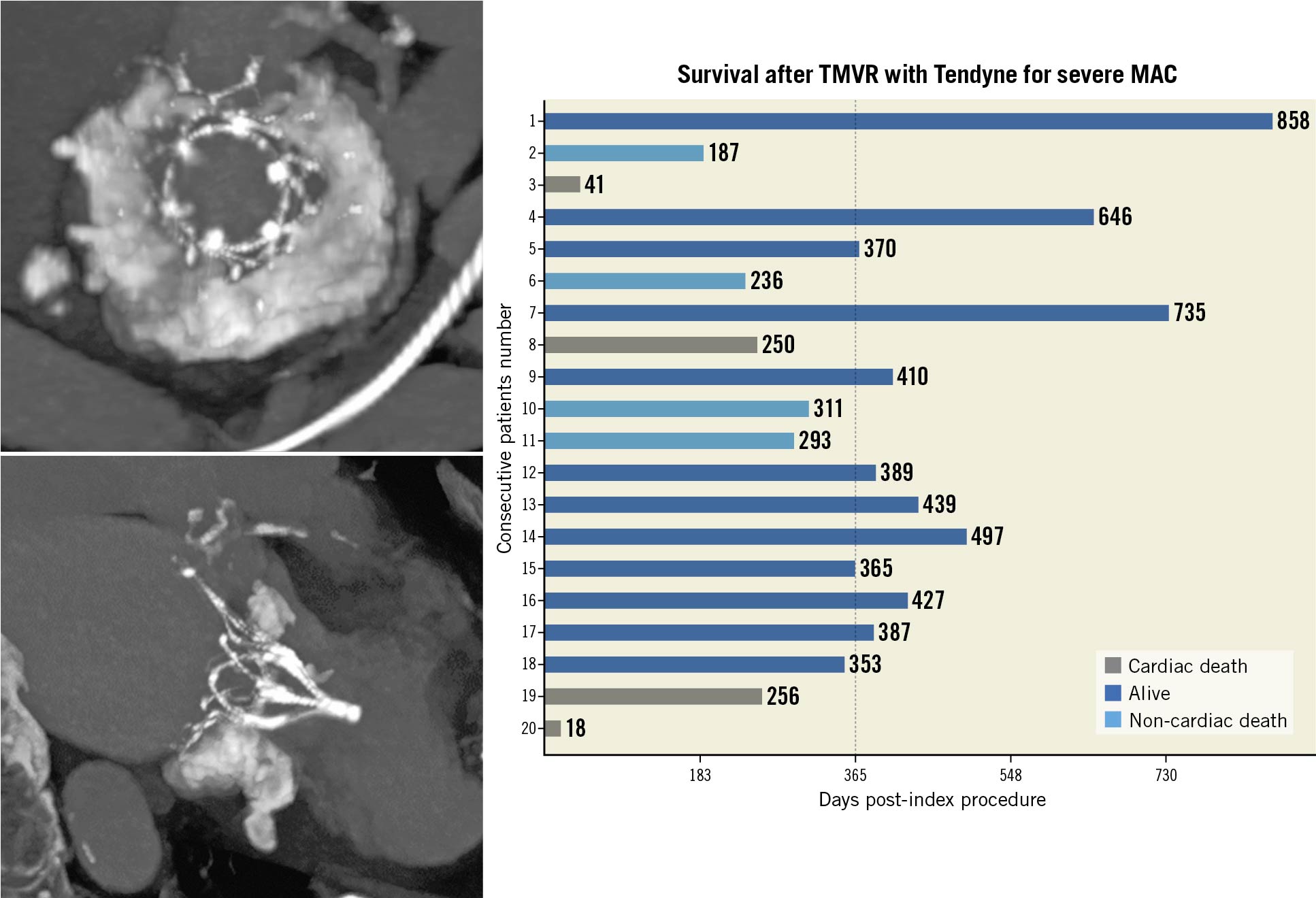
Median follow-up for the entire study cohort was 368 days (range 18 to 858 days) (Figure 4). Death at one year occurred in eight of the 20 patients, including four cases that were cardiovascular-related. One patient, who experienced acute mesenteric ischaemia on post-operative day 16 and haemorrhagic shock following exploratory laparotomy, died 18 days post-index procedure; the CEC adjudicated the event as related to both the device and procedure. One patient died at home due to worsening congestive heart failure and coronary artery disease on post-operative day 250. One patient experienced sudden cardiac arrest at home 256 days post-index procedure. One patient died of acute systolic heart failure 41 days post-index procedure. There were four non-cardiac deaths. One patient was hospitalised for pneumonia 299 days post-procedure, developed sepsis and passed away 12 days later. Three patients treated under CU experienced non-cardiac death, including two by suicide and one related to renal insufficiency 269 days post-procedure. Up to one year, of the six patients hospitalised for heart failure, three died after hospitalisation. The cause of death following hospitalisation was described previously as cardiac arrest, heart failure and suicide.
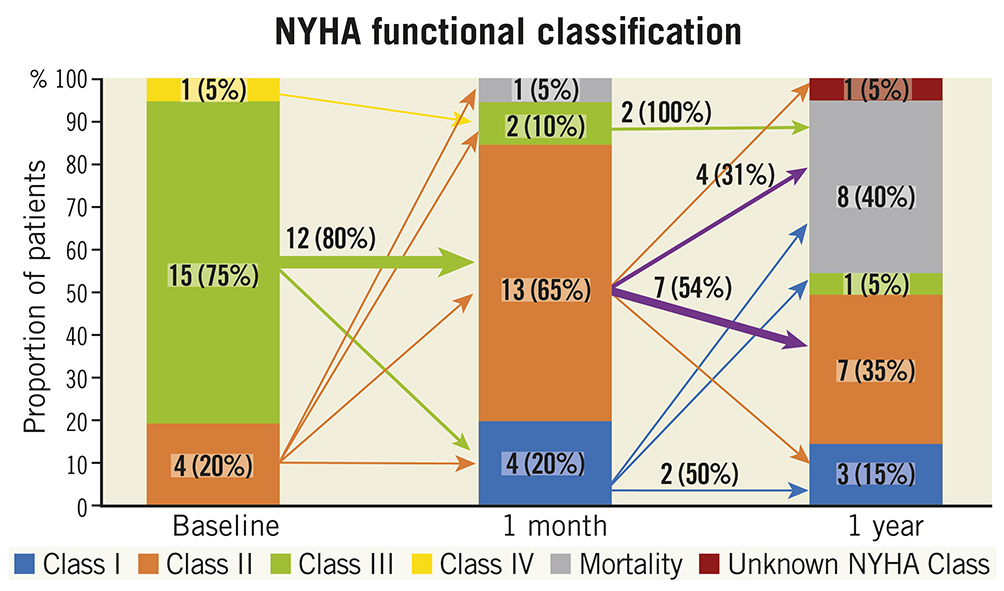
Figure 4. New York Heart Association Functional Class at baseline, one-month and one-year follow-up. The arrows indicate the absolute and proportion of patients moving between classes within follow-up time points. A wider arrow size suggests the majority of that subgroup moving to a different class. NYHA: New York Heart Association
One patient experienced a non-disabling stroke (5%) intraprocedurally, with symptoms that resolved by the one-month follow-up. At the one-month visit the patient was hospitalised for heart failure with haemolysis; a paravalvular leak (PVL) was identified, which was resolved two months later with a vascular plug. There were no other mitral re-interventions or surgical procedures performed up to one year. There were no other instances of PVL, and the MR grade was none or trivial in all patients at one year. There were five device- or procedure-related bleeding events: one, previously described above, due to mesenteric ischaemia and haemorrhagic shock, three procedural bleeding events resolved with transfusion or iron supplements, and one due to supratherapeutic international normalised ratio (INR) associated with the anticoagulation regimen that resolved with dosage change. There were two reported events of new onset atrial fibrillation: one began during the administration of anaesthesia, and one was successfully cardioverted. At one year, echocardiographic imaging demonstrated sustained relief of MR, with no prosthetic dysfunction or stenosis. Overall, these results demonstrated device success, as defined by MVARC-2 criteria, in 11 of 12 patients (92%), with one patient experiencing both a non-disabling stroke and requiring post-procedural mitral re-intervention for PVL.
Among the 12 survivors at one year, 11 patients (92%) were in NYHA Functional Class I or II (Figure 5). Paired analyses for Kansas City Cardiomyopathy Questionnaire (KCCQ) quality-of-life assessments were available in seven survivors. Among these individuals, at one year the mean improvement in KCCQ score was 29.9±26.3 with an increase ≥10 points in five (71.4%) patients. Paired analyses for six-minute walk tests (6MWT) were available for only two MAC FS participants, as some in-person clinic visits were missed due to the COVID-19 pandemic.
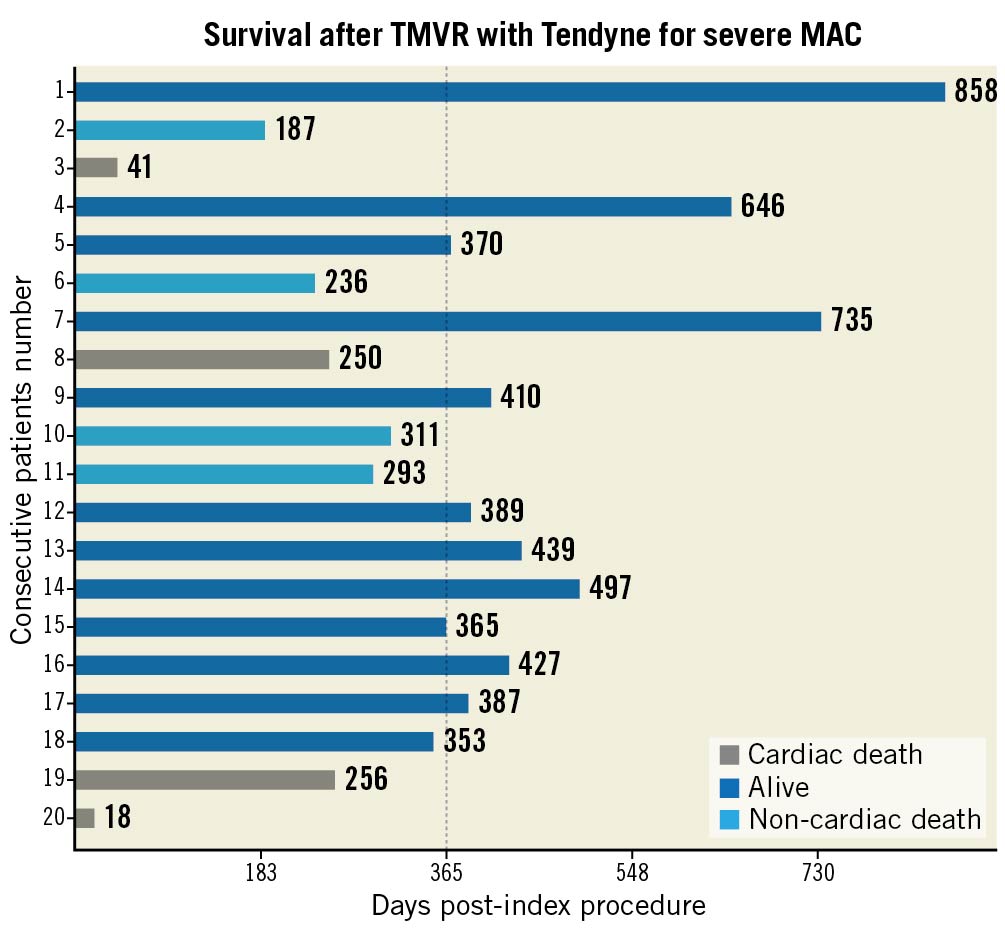
Figure 5. Survival after TMVR with the Tendyne for severe MAC. Follow-up time for the 20 patients with patients listed on the y-axis in descending order of treatment. The x-axis indicates duration of last follow-up. Dotted line indicates time mark at one-year follow-up. MAC: mitral annular calcification; TMVR: transcatheter mitral valve replacement
Discussion
The early success of Tendyne as a dedicated TMVR device for the CU treatment of patients with severe MAC was an important milestone for the therapy6. The Tendyne MAC Feasibility Study was initiated to study the outcomes of TMVR in these patients further. In this study of Tendyne TMVR in severe MAC, there were high rates of technical success (95%), elimination of MR (100%), and safety (i.e., no procedural mortality; 30-day mortality 5%), with durability of the prosthesis and clinical improvement in the majority of patients during follow-up. These data further expand on the early experience of Tendyne TMVR for severe MAC, with results that represent considerable improvements in outcomes for this challenging patient subset, particularly in comparison to prior experiences with off-label devices45.
Patients with symptomatic mitral disease and severe MAC are common and represent a therapeutic challenge due to the surgical risks of atrioventricular groove rupture or myocardial tearing, as well as the frequent presence of adverse comorbidity. Success may be attributable to several design features of the Tendyne TMVR device, including a valve designed specifically for the mitral anatomy, avoiding the need for rapid ventricular pacing and, if needed, the ability to reposition and retrieve the valve. Specific to patients with severe MAC, the Tendyne apical anchor minimises the requirement to achieve valve securement at the annulus where the MAC burden is located. In addition, the anatomically shaped design of the outer frame readily conforms to rigid MAC while the inner frame remains functional. In this early experience, there has been no evidence of bioprosthetic valve dysfunction during follow-up.
In this study, the sample size (n=20) was relatively small due to the novelty of Tendyne TMVR for this application. Nevertheless, some insight may be garnered from the mortality rates observed (Central illustration). The 30-day all-cause mortality rate (5%) was relatively low for these patients who would otherwise be at high risk for conventional surgery, reflecting a highly favourable procedural safety profile for the device. However, death due to any cause at one year occurred in eight patients (40%). The association of severe, non-cardiac morbidity with the presence of MAC has been described previously and was also noted in this study cohort111213. Four of the eight deaths were non-cardiac, including two suicides. The resulting rate of cardiac mortality of 20% at one year equals that of high-risk transcatheter edge-to-edge repair studies (reported cardiac mortality rate 16-22%)14 and compares favourably to SAPIEN in MAC registries (reported cardiac mortality rate 24-33%)415. Consideration of the impact on life expectancy of morbidity beyond that attributable to mitral disease remains important, as with other methods for assessing benefit and futility in transcatheter and surgical therapy16. We believe the technical success demonstrated in the study could translate into improved long-term outcomes when such considerations are undertaken. Due to this early technical success, the Tendyne MAC FS closed enrolment on 30 October 2019 and a dedicated MAC cohort was initiated for the Tendyne SUMMIT pivotal clinical trial that remains ongoing (https://clinicaltrials.gov/ct2/show/NCT03539458).
Central illustration. One-year outcomes of Tendyne in MAC. Our study included 20 patients with severe MAC and high surgical risk undergoing Tendyne in MAC (two representative cross-sectional and longitudinal contrast-enhanced cardiac computed tomography images of a Tendyne prosthesis in MAC are depicted). All-cause death at one year occurred in eight (40%), cardiac death, however, occurred in only four of the 20 patients (20%). MAC: mitral annular calcification; TMVR: transcatheter mitral valve replacement
Presently, conventional risk models (e.g., STS-PROM, EUROSCORE II) do not incorporate MAC anatomy for prognostication; such anatomy must be carefully considered for determining TMVR feasibility. Balloon valvuloplasty (e.g., 26 mm to 30 mm with True or True Flow [Bard] or Inoue-Balloon [Toray]), performed either transeptally or transapically, was used in the majority of patients (11 of 20 cases). Typically, balloon valvuloplasty was considered when there was leaflet restriction, mitral stenosis, or if a calcified spicule(s) extended towards the left ventricular cavity, and when these features posed risk for lack of inner frame expansion. One patient, who had a calcific spicule protruding >3 mm, did not undergo a balloon valvuloplasty; initially, the valve did not completely expand and was partially recaptured. The valve was then repositioned, with confirmation by intraprocedural transoesophageal echocardiography and fluoroscopy, and deployed successfully. These observations demonstrate the need for comprehensive multimodality imaging for preprocedural and procedural assessments, as well as the utility of a recapturable and repositionable prosthesis.
Our encouraging midterm results from the CU and early feasibility studies of Tendyne TMVR are early evidence in support of a transcatheter solution for high-risk patients with severe MAC, many of whom are at prohibitive surgical risk111. In this challenging subset of patients, Tendyne TMVR potentially offers a less-invasive therapeutic option with sustained echocardiographic and clinical success.
Our results also emphasise the need for a thorough patient selection process involving a multidisciplinary Heart Team for this high-risk subset of patients. Our data do not allow strong conclusions regarding the possible exclusions of patient subsets due to possible futility; however, age, pre- and post-procedure rehabilitation potential and the ability to follow guideline-directed medical therapy are key factors in this decision-making process.
Study limitations
Due to the COVID-19 pandemic, some in-person follow-up assessments were unable to be performed. Overall, seven patients could not complete a 6MWT at the one-year window due to the pandemic, though follow-up was completed on all patients remotely. While the sample size was limited, the reason for this limitation was the high technical success observed in these patients, which led to cessation of the feasibility study and then initiation of the ongoing pivotal clinical trial (NCT02321514).
Conclusions
TMVR in severe MAC with a dedicated prosthesis shows encouraging midterm outcomes, with sustained MR elimination and clinical improvement up to one year. The observed high all-cause mortality rate in this high-risk patient population highlights the need for a thorough patient selection process in a multidisciplinary Heart Team approach.
Impact on daily practice
Patients with severe MAC and MR are considered high surgical risk, hence they are often denied further interventions. The here presented midterm data of TMVR with the Tendyne device in patients with severe MAC and MR reflect the high-risk population but also provide evidence that TMVR with the Tendyne is a feasible and durable option with excellent acute and acceptable midterm clinical outcomes. Dedicated TMVR therapy with the Tendyne device may therefore have a future role in these anatomically challenging high-risk patients.
Funding
The present report is an analysis of 20 patients treated with Tendyne, either through global compassionate use (CU) or as part of The Feasibility Study of the Tendyne Mitral Valve System in Mitral Annular Calcification (NCT03539458, i.e., the Tendyne MAC Feasibility Study [MAC FS]) sponsored by Abbott).
Conflict of interest statement
M. Gössl reports consulting for Abbott Vascular, and is Chair of the Subject Eligibility Committee for the SUMMIT trial. V. Thourani reports consulting and research for Abbott Vascular. V. Babaliaros reports consulting for Abbott (less than $5,000), and Edwards Lifesciences. L. Conradi reports being a member of the advisory board for Abbott Vascular, and being a consultant for Boston Scientific, Edwards Lifesciences, and Medtronic. B. Chehab reports being a member of the advisory board and a consultant for Abbott Structural, Edwards Lifesciences, and Medtronic. N. Dumonteil reports consulting and proctoring fees for Abbott Vascular, Ancora Heart, Boston Scientific, Edwards Lifesciences and Medtronic. V. Badhwar reports institutional research support from and being a consultant (non-remunerative) for Abbott. D. Rizik reports being on the Medical Advisory Board of Abbott Vascular, and being on the Executive Physician Council of Boston Scientific. B. Sun reports being a consultant and clinical proctor for Abbott Vascular and being a selection committee member for the Tendyne Device (Summit Clinical Trial). R. Bae reports consulting for Abbott Vascular - Speakers Bureau. R. Guyton reports being a consultant for Edwards Lifesciences (no consultation or compensation in last 12 months). M. Chuang is an employee of the Beth Israel Deaconess Medical Center Cardiovascular Imaging Core Laboratory which received funds from the study sponsor to perform image analyses. He also reports Echo Core Laboratory services for Abbott Vascular. P. Blanke reports being a consultant for Edwards Lifesciences and W.L. Gore, CT Core Laboratory services for Abbott Vascular, institutional core laboratory services for Abbott Laboratories, related to this manuscript, with no direct personal compensation, and institutional core laboratory services not related to this manuscript for Medtronic, Edwards Lifesciences, Boston Scientific, and Neovasc without direct personal compensation. P. Sorajja reports consulting or advisory board participation for Abbott Structural, Anteris, Boston Scientific, Medtronic, NeoVasc, TriFlo, VDyne, and W.L. Gore.
Supplementary data
To read the full content of this article, please download the PDF.

