
Abstract
Aims: Longitudinal outcomes of transcatheter therapies for secondary mitral regurgitation (MR) have been variable. This study examined predictors of one-year outcome following transcatheter mitral valve implantation (TMVI) with the Tendyne device.
Methods and results: The first 100 consecutive patients with moderate-severe or severe MR enrolled in the Tendyne CE Mark trial were examined. Multivariable analyses assessed the impact of preoperative clinical and echocardiographic characteristics on one-year freedom from death or heart failure hospitalisation (HFH). All 100 patients underwent Tendyne TMVI without operative mortality. Univariate analysis was performed on implanted subjects, followed by multivariate analysis in those with complete predictive variable data. Patient characteristics: 76.5% male, 60.8% NYHA Class III/IV, age 75.6±7.5 years and Society of Thoracic Surgeons predicted risk of mortality of 8.5±6.1%. Increased left ventricular end-diastolic dimension was associated with one-year Tendyne benefit following univariate analysis (OR 0.35, p=0.010). Following multivariable adjustment, only severe MR, defined as a composite of effective regurgitant orifice area ≥0.3 cm2 or regurgitant volume ≥45 ml, was associated with freedom from death or HFH at one year (OR 0.16, p=0.032).
Conclusions: Preoperative severe MR was predictive of improved one-year outcome following Tendyne TMVI. These results may inform therapy selection for the management of secondary MR and left ventricular dilatation.
Introduction
The current management of mitral regurgitation (MR) has been informed by evidence of both the deleterious effects of its natural history and the benefit to longitudinal outcome with successful MR correction1,2. For patients at high/prohibitive surgical risk, transcatheter therapies have emerged as a promising option3,4,5. While surgical repair has proven to be a durable and effective standard of care for patients with primary MR, surgical correction of severe secondary MR has not been shown to impact on survival6. Mitral valve replacement is effective in eliminating MR and remains durable in secondary MR. Recent experience with transcatheter mitral valve implantation (TMVI) with the Tendyne device (Abbott Vascular, Santa Clara, CA, USA) has shown favourable early results in patients with high/prohibitive surgical risk5,7,8.
Recent randomised evidence of transcatheter edge-to-edge repair of secondary MR has been encouraging to reduce late death and heart failure hospitalisations (HFH) in specific risk groups9,10. However, for patients at the extremes of disease, such as moderate-severe or severe MR and ventricular dilatation with reduced ejection fraction, outcomes of transcatheter edge-to-edge repair have been questionable, thus introducing a potential therapeutic and knowledge gap9,10,11,12,13,14.
The objective of this study was to examine the one-year results of the first 100 patients receiving TMVI with the Tendyne prosthesis to ascertain potential predictors of freedom from death and HFH.
Methods
STUDY COHORT
The CE mark global feasibility clinical study of the Tendyne Transcatheter Mitral Valve System is a single-arm open-label clinical trial that commenced in November 2014 and continued with expanded enrolment (NCT02321514). Patients included in the study were those with symptomatic severe MR per Mitral Valve Academic Research Consortium guidelines with ambulatory NYHA Class II or greater functional class and whom the institutional Heart Team have assessed as not being suitable candidates for surgical treatment. Those with severe mitral stenosis, severe mitral annular calcification, prior mitral or aortic valve intervention, or a chest condition that prevents transapical access were excluded. Anatomic or haemodynamic exclusions were left ventricular end-diastolic diameter (LVEDD) >70 mm, fixed pulmonary hypertension (pulmonary artery systolic pressure ≥70 mmHg), severe tricuspid regurgitation, severe right ventricular dysfunction, left ventricular ejection fraction (LVEF) ≤30%, or core lab adjudicated size and left ventricular outflow tract (LVOT) exclusions based on transoesophageal echocardiography (TOE) and cardiac computed tomography (CT) (Figure 1). The present study examined the first 100 consecutive patients receiving TMVI with the Tendyne device to assess preoperative conditions associated with clinical outcome at one year after implantation.
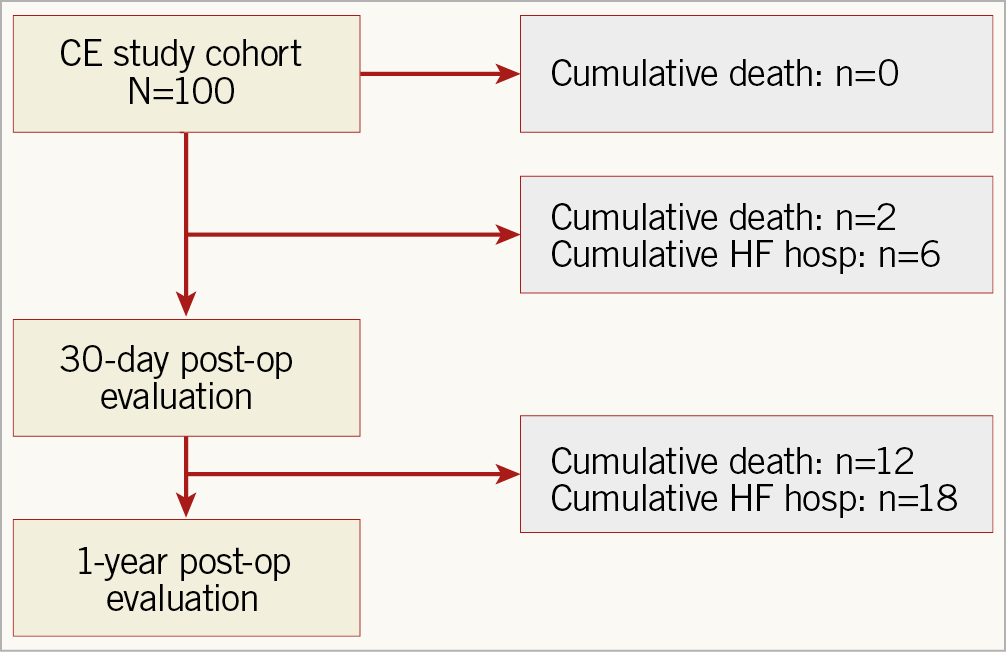
Figure 1. CONSORT study flow diagram.
IMPLANTATION TECHNIQUE
Tendyne implantation is performed under general anaesthesia using a 4-6 cm left anterolateral thoracotomy centred over the left ventricular apex that is anatomically predicted by preoperative CT and confirmed with intraoperative surface echo and TOE. The apex is prepared coaxial to the mitral orifice, a 0.035” guidewire is delivered across the mitral valve under TOE guidance, a 36 Fr delivery sheath is delivered across the valve and the self-expanding Tendyne prosthesis is deployed and positioned within the mitral orifice with the prominent portion of the cuff oriented along the aorto-mitral continuity. The delivery sheath is then removed, and the prosthesis is secured to anatomic stability with tension on the polyethylene tether to manufacturer and haemodynamic parameters and affixed with an epicardial pad.
FOLLOW-UP EVALUATION
Patients were maintained on oral warfarin for a minimum of three months or indefinitely if patients were in atrial fibrillation. Clinical follow-up occurred at 1, 3, 6 and 12 months and annually thereafter. Symptoms were objectively evaluated by 6-minute walk distance, Kansas City Cardiomyopathy Questionnaire (KCCQ) and NYHA functional class assessments. Echocardiograms were performed at 1, 3, 6 and 12 months and annually thereafter and prostheses were evaluated using Mitral Valve Academic Research Consortium criteria15,16. Core lab adjudicated performance thresholds included central or paravalvular MR <1+, mitral valve gradient <6 mmHg and effective orifice area >1.5 cm2.
STUDY ENDPOINTS
The primary safety endpoint of the CE mark global feasibility study was the 30-day composite outcome of device success and freedom from cardiovascular death, reintervention for valve-related dysfunction, stroke, myocardial infarction, major bleeding or vascular complications, and new dialysis. Device success was defined as freedom from mortality, stroke and need for subsequent surgical or interventional procedures related to either access or the device. The secondary safety endpoint was a composite of device success and freedom from device- or procedure-related serious adverse events evaluated at two years post procedure.
In the current analysis, the composite endpoint used to assess clinical response to Tendyne TMVI was freedom from death or HFH one year after implantation.
STATISTICAL ANALYSIS
The preoperative baseline clinical and echocardiographic characteristics of the study population were evaluated for predictive ability of death or HFH following Tendyne implantation. In total, 41 clinical and echocardiographic variables were assessed, which included: Tendyne valve profile implanted, age, LVEF, LVEDD, left ventricular end-diastolic volume index (LVEDVi), prior percutaneous coronary intervention (PCI) and Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) for surgical mitral valve replacement. Thresholds for advanced disease included LVEDVi ≥96 ml/m2, preoperative NT-proBNP level ≥1,600 pg/ml, and severe MR defined as a composite of effective regurgitant orifice area (EROA) ≥0.3 cm2, or regurgitant volume ≥45 ml1.
A sub-cohort was identified with complete data collection for all variables and univariate regression was applied to develop a multivariate prediction model. Univariate logistic regression was performed for each variable to analyse their association with one-year outcome using open source R statistical programming language, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Variables with p≤0.2 were eligible for inclusion in the multivariate model. Correlation and multicollinearity among parameters were addressed, and an acceptable base model was determined by variance inflation factors and Akaike Information Criterion for relative fit of the model17. Finally, stepwise regression was performed on the base model within the sub-cohort to identify predictors of one-year clinical outcome.
ETHICS STATEMENT
There were 24 centres participating in this study, 3 in Australia, 8 in Europe and 13 in the USA. At each enrolling site, institutional review board approval and unique patient consent was obtained.
Results
All 100 patients underwent Tendyne TMVI procedure without operative mortality. Univariate analysis was performed on the complete cohort with the Tendyne valve successfully implanted (N=97) (Figure 1). Multivariate analysis was performed in a sub-cohort of 51 patients with complete data collection of predictive variables by univariate analysis (Supplementary Table 1). The patients were 76.5% male with an age of 75.6±7.5 years. NYHA Class III/IV functional classification was present in 60.8%. The STS PROM for mitral replacement was 8.5±6.1.
Forty-one clinical and echocardiographic characteristics underwent univariate logistic regression analysis that identified 21 variables predictive for the study endpoint of having an impact on one-year mortality and HFH (Table 1). Of these, nine factors reached statistical significance. Higher one-year mortality and HFH event rates were observed in patients with elevated age (OR 1.09, p=0.004), renal insufficiency (eGFR <60 ml/min/1.73 m²) (OR 2.85, p=0.017), prior PCI (OR 3.31, p=0.006) and those with a history of hypertension (OR 4.06, p=0.014). Conversely, lower one-year event rates were observed in patients with a larger body surface area (OR 0.16, p=0.042), severe MR (OR 0.20, p=0.016) and a larger LVEDD (OR 0.35, p=0.010).
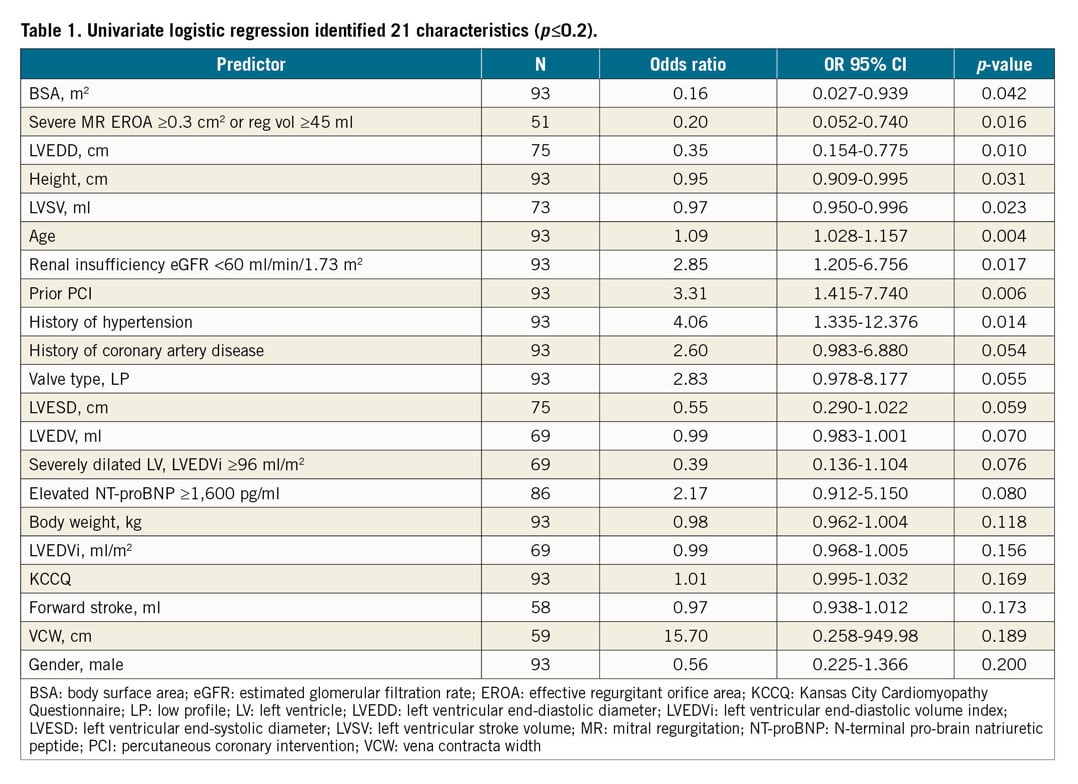
Multicollinearity adjustment and Akaike Information Criterion refined the multivariable model to include 11 significant predictors: age, gender, body surface area, renal insufficiency, history of hypertension, coronary artery disease, prior PCI, LVEDD, standard or low valve profile Tendyne prosthesis, elevated NT-proBNP, and the presence of severe MR – defined as a composite of EROA ≥0.3 cm2 or regurgitant volume (RVol) ≥45 ml.
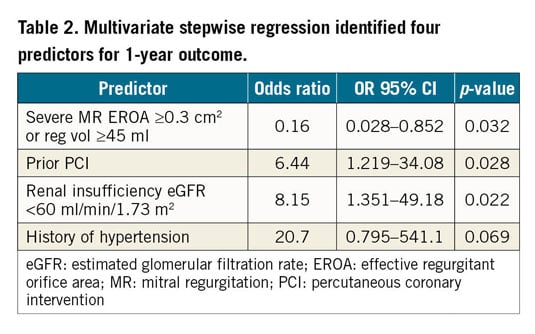
Following multivariate logistic regression analysis (Table 2), increased composite one-year mortality and HFH accompanied patients with prior PCI (OR 6.44, p=0.028), renal insufficiency (OR 8.15, p=0.021), and history of hypertension (OR 20.7, p=0.069). Lower composite one-year mortality and HFH was associated with those with preoperatively severe MR (OR 0.16, p=0.032).
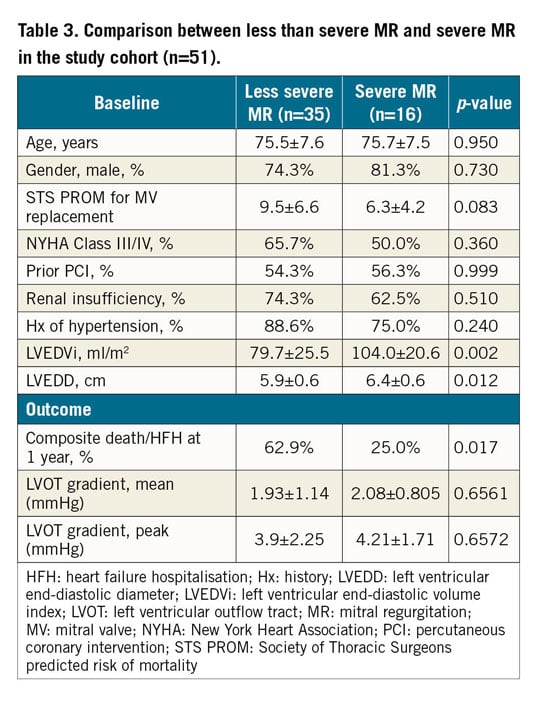
Less than severe MR and severe MR groups were then compared (Table 3). Patients in the severe MR versus the less than severe MR group had increased LVEDD (p=0.012) and LVEDVi (p=0.002) and less composite one-year mortality and HFH (p=0.017).
Discussion
This analysis of the first 100 Tendyne TMVI procedures in the CE mark global feasibility study defined predictors of composite one-year outcomes of mortality and HFH. Though large baseline ventricular size and the presence of severe MR were found to be statistically significant univariate predictors of improved one-year outcome following Tendyne TMVI, only severe MR was determined to be a significant multivariate predictor. Preoperative renal insufficiency (eGFR <60 ml/min/1.73 m2), history of hypertension, and prior PCI were found to be significant predictors of increased mortality and heart failure readmissions at one year.
Implantation of the Tendyne transapical TMVI prosthesis has been shown to be safe and highly effective for patients at prohibitive surgical risk, and early experiences have facilitated an expansion of adjunctive techniques and applications7,8,18,19,20,21. Procedural mortality for the high-risk cohort receiving Tendyne TMVI remains zero with echocardiographic results of MR <1+. In a recent report of the first 100 patients enrolled in the Tendyne global feasibility trial8, the mean follow-up was 13.7 months with a one-year freedom from all-cause mortality of 72.4%. Of the 26 deaths during follow-up, 22 were deemed cardiac deaths, seven from heart failure. Importantly, 88.5% of survivors were in NYHA functional Class I or II at one year. In the current study, the composite of one-year mortality and HFH was assessed to determine if preoperative clinical and echocardiographic characteristics might predict outcomes. Forty-one preoperative characteristics were assessed, 21 of which were identified by univariate analysis (p≤0.2) as having an impact on composite outcome; only LVEDD and severe MR were associated with significantly lower one-year event rates. Intriguingly, larger LVEDD was associated with positive clinical outcomes, which may be a function of the Tendyne system’s unique tether and apical pad. However, following multivariate regression, only severe MR was found to be predictive of improved outcomes.
Though inclusion criteria for the Tendyne global feasibility study were agnostic to the mechanism of MR, 90% of implants were for secondary MR. The presence of LV dilatation was common (LVEDD 6.0±0.7, IQR 5.7-6.6 cm, LVEDV 168.2±52.9, IQR 131-209 mL). The current randomised clinical trial to evaluate the safety and effectiveness of using the Tendyne Mitral Valve System for the treatment of symptomatic mitral regurgitation (SUMMIT) focuses on patients with a principal mechanism of secondary functional MR22. Two recent randomised clinical trials on transcatheter mitral valve repair with the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) examined the treatment of secondary functional MR with very different results, raising significant questions as to which patients may benefit from intervention9,10.
The cardiovascular outcomes assessment of the MitraClip percutaneous therapy for heart failure patients with functional mitral regurgitation (COAPT) trial only enrolled patients who were optimised with guideline-directed medical therapy (GDMT) for three months who were then randomised to MitraClip + GDMT versus GDMT alone9. Importantly, patients with advanced ventricular dilatation and those with an LVEF <20% were excluded. The MitraClip group had a substantial reduction in all-cause mortality at 24 months versus GDMT (29.1% vs 46.1%, HR 0.62, p=0.001). Inferior outcomes of the MitraClip group were observed in a subset of patients with larger ventricles and less than severe MR. The multicentre study of percutaneous mitral valve repair MitraClip device in patients with severe secondary mitral regurgitation (MITRA-FR) trial similarly randomised patients to MitraClip + GDMT versus GDMT alone10. However, MITRA-FR permitted more advanced degrees of ventricular dilatation, GDMT at baseline and follow-up was determined by the local Heart Team instead of a central governing body, and the primary composite endpoint of death and unplanned hospitalisation was at 12 months. This study concluded that the rate of death or unplanned hospitalisation for HF at one year did not differ significantly between patients who underwent MitraClip + GDMT versus those who received GDMT alone. Though there are significant differences in these trials, their similarities highlight the fact that, in patients with worse ventricles and less than severe MR, transcatheter mitral repair may not be the ideal therapy. A pathoanatomic assessment of patients incorporating annular dilation, ventricular size and LVEF as well as leaflet tethering may be helpful to determine which patient may be better served by surgery, transcatheter repair or TMVI13. The current study supports the evolving paradigm that, in secondary MR, valvular interventions may provide clinical benefit for patients with more severe MR. However, following TMVI with the Tendyne device, univariate analysis showed that larger LV dimensions were actually associated with lower rates of death or hospitalisation, though this did not reach significance in the multivariable model. Importantly, higher event rates did not appear to be related to outflow tract obstruction in smaller ventricles as the post-implantation LVOT gradients in patients with smaller and larger ventricles were similar (Supplementary Table 2). These unique findings of Tendyne TMVI benefit in patients with larger ventricles and severe MR appear congruent to the concept of potential intervention benefit in patients with disproportionate MR and large LV size23. Therefore, Tendyne TMVI may address a therapeutic gap existing for patients with severe LV dilatation where suboptimal results may be observed with transcatheter MV repair.
Limitations
This study has several limitations. The Tendyne CE mark global feasibility study was a multicentre non-randomised single-arm trial to examine the safety and early feasibility of this TMVI device; it was not designed to demonstrate benefit over an alternative therapy. While all participating sites were accomplished mitral centres, all operators were new to the implant technique and thus operator experience could not be controlled for. Following univariate identification of one-year predictors in the current study, the methodological rigour of the multivariate model required patients to have baseline measurement of candidate predictors and known one-year outcome. The strict methodology of this study required complete transthoracic echocardiographic data to be available for accurate assessment of predictors in the univariate and multivariate analysis. As such, patients with incomplete echocardiographic variables were excluded from this portion of the analysis. However, 30-day and one-year results of the study population (n=51) were quite representative of the total cohort (n=100) (Supplementary Table 3).
Conclusions
In conclusion, the Tendyne TMVI remains a safe and highly effective procedure for the management of patients with MR who are deemed to be at prohibitive surgical risk. Risk-adjusted predictors of improved one-year composite outcome of mortality and HFH include preoperative severe MR, while prior PCI, history of hypertension, and renal insufficiency are associated with worse one-year outcome.
|
Impact on daily practice Tendyne TMVI applied to patients with prior PCI, history of hypertension, and renal failure results in higher rates of one-year mortality or heart failure hospitalisation. Conversely, Tendyne applied to those with severe MR results in improved one-year outcomes, with a trend to similar results in patients with more left ventricular dilatation. These results may inform the therapeutic gap raised by recent transcatheter mitral repair trials that highlight worse outcomes in patients with severe LV dilatation. |
Acknowledgements
The co-authors would like to thank Lihua Li and Robert McNutt for superb assistance in data accrual and analysis.
Funding
The Tendyne global feasibility study was supported by Abbott.
Conflict of interest statement
P. Sorajja and U. Schaefer serve as consultants for Abbott, Medtronic, Boston Scientific, and Edwards. V. Thourani, P. Grayburn and V. Babaliaros serve as consultants for Abbott and Edwards. J. Leipsic, M. Chuang and D. Muller serve as consultants for Abbott. V. Badhwar serves as a consultant for Abbott (uncompensated). P. Blanke is a consultant for Abbott. The other authors have no conflicts of interest to declare.

