
Abstract
Aims: Transcatheter mitral valve replacement (TMVR) is a novel technique for treating mitral regurgitation. We report 18-month/two-year outcomes of the five first-in-man patients undergoing chronic TMVR with the Tendyne device.
Methods and results: Five patients (STS score range 14%-23%) underwent TMVR using the Tendyne system under compassionate use at a single centre. One patient (who was non-compliant with anticoagulation) died nine months after TMVR following a cerebrovascular accident. Whilst some of the remaining patients experienced recognised post-TMVR complications (left ventricular outflow tract obstruction, paravalvular leak, stent thrombosis), all patients alive at 18 months/two years after TMVR reported significant reduction in NYHA class and increase in exercise capacity compared to baseline. Transthoracic echocardiography demonstrated valve stability, absent transvalvular mitral regurgitation, and significant reductions in tricuspid regurgitation severity and systolic pulmonary arterial pressure.
Conclusions: Eighteen months/two years after compassionate use of the Tendyne system in a high-risk surgical group, the majority of patients were alive, patient symptoms improved, and the device was stable with good haemodynamic function, no late migration and no new paravalvular leak. Continued follow-up of patients treated with the Tendyne system is required in order to describe its safety and efficacy further.
Abbreviations
3DTOE: three-dimensional transoesophageal echocardiography
6MWT: 6-minute walk test
AF: atrial fibrillation
CABG: coronary artery bypass graft
EuroSCORE: European System for Cardiac Operative Risk Evaluation
IABP: intra-aortic balloon pump
LVEDD: left ventricular end-diastolic dimensions
LVEF: left ventricular ejection fraction
LVESD: left ventricular end-systolic dimensions
LVOTO: left ventricular outflow tract obstruction
LV-SV: left ventricular stroke volume
MSCT: multislice computed tomography
MR: mitral regurgitation
NYHA: New York Heart Association
PASP: pulmonary artery systolic pressure
PVL: paravalvular leak
STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality
TAVI: transcatheter aortic valve implantation
TMVR: transcatheter mitral valve replacement
TTE: transthoracic echocardiography
TR: tricuspid regurgitation
VA-ECMO: venous-arterial extracorporeal membrane oxygenation
Introduction
Mitral regurgitation (MR) is a leading cause of valvular heart disease1; however, up to half of patients with moderate to severe MR are not referred for conventional mitral valve surgery on the basis of advancing age or multiple comorbidities2,3. This has led to a surge in the development of transcatheter mitral valve techniques that do not require cardiopulmonary bypass or sternotomy. Transcatheter mitral valve repair with the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA) is an alternative treatment for a subset of patients with MR at prohibitive operative risk4, but some patients may not be anatomically or technically suitable for this intervention. Alternative novel transcatheter-based technologies to treat MR are under development5-8, with transcatheter mitral valve replacement (TMVR) being one such group of techniques. However, its development has been hindered by the complex structure and function of the mitral valve – the D-shaped annulus, changes in annular shape within the cardiac cycle, and variability of leaflet and subvalvular apparatus anatomy. Due to the complexity of mitral valve anatomy and the dynamic mitral environment, potential complications of TMVR include device migration, paravalvular leak (PVL), left ventricular outflow tract obstruction (LVOTO), and impingement of the circumflex coronary artery or coronary sinus9. The Tendyne device (Abbott, Abbott Park, IL, USA) is a custom-made, catheter-based technology for the treatment of severe MR of varying aetiology (degenerative or functional). Our group has reported periprocedural and 30-day results of the first-in-human implants with the Tendyne device10. In the current study, we report long-term outcomes of the first-in-man compassionate use implants with the Tendyne device.
Methods
PATIENTS
Five first-in-man chronic implant patients underwent TMVR using a Tendyne system under compassionate use at The Royal Brompton Hospital, London, UK, four patients between October 2014 and January 2015 (with two-year follow-up), and a fifth in September 2015 (with 18-month follow-up). Patient demographics are summarised in Table 1. Patient #3 presented with severe primary MR (mitral valve prolapse secondary to chordal rupture), and the remaining four patients presented with severe secondary MR (two had previously undergone coronary artery bypass graft surgery [CABG], one had previously undergone mitral valve repair with a 38 mm Carpentier-Edwards Physio II annuloplasty ring [Edwards Lifesciences, Irvine, CA, USA], and one had ischaemic cardiomyopathy secondary to anterior myocardial infarction). All had multiple hospital admissions with decompensated heart failure and all presented in New York Heart Association (NYHA) Class III/IV despite optimal medical therapy. Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) scores ranged between 6% and 23%, and European System for Cardiac Operative Risk Evaluation (EuroSCORE II) scores ranged between 3% and 30%. All patients were assessed clinically by our transcatheter valve and heart failure teams, and all either were deemed high-risk or declined conventional mitral valve surgery. All four patients with secondary MR were considered anatomically unsuitable for any other CE-marked device available at the time of assessment, including MitraClip, and the patient with posterior mitral valve prolapse declined MitraClip intervention. Exclusion criteria for the compassionate use of the Tendyne device were based on mitral annular sizing (<34 mm or >50 mm intercommissural diameter). All five patients met inclusion criteria for compassionate use (extreme risk for surgery, >grade 3 MR, NYHA Class ≥II) and all were deemed technically suitable for TMVR on a UK compassionate use protocol. The study was approved by the Institutional Committee on Human Research at The Royal Brompton Hospital, and all patients gave informed consent to participate.
PREPROCEDURAL IMAGING AND SCREENING
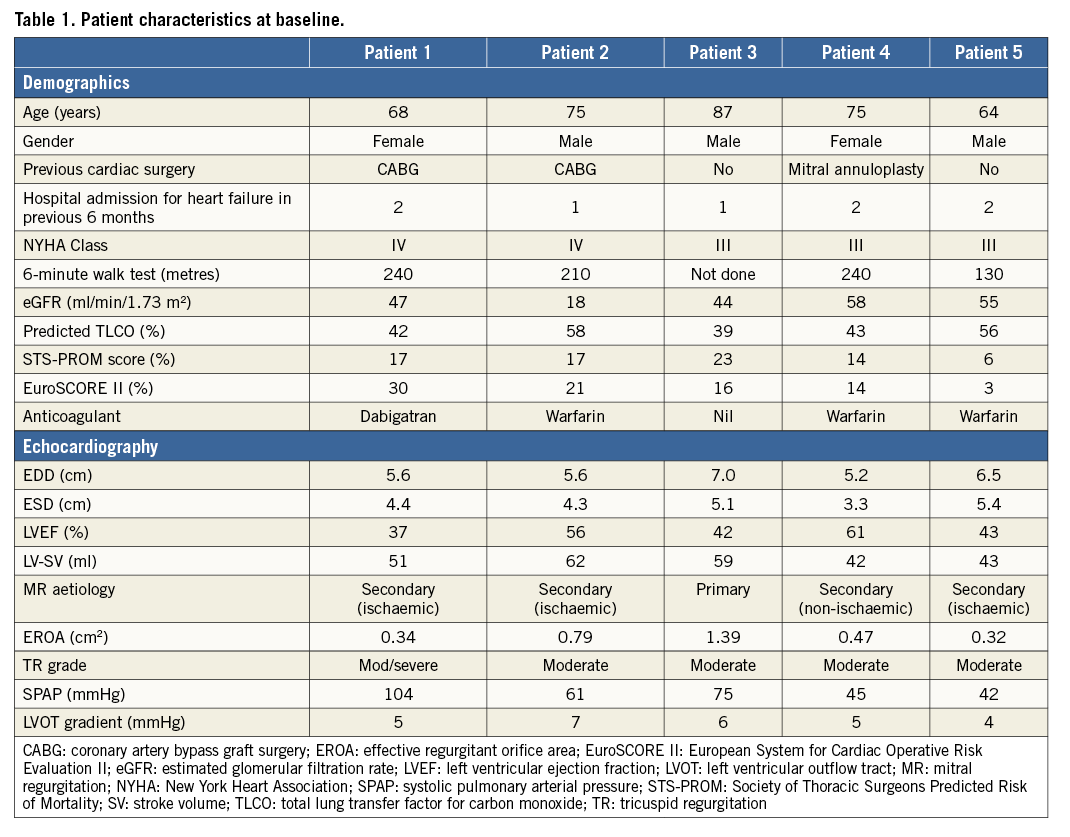
All patients who were being considered for TMVR in our institution underwent preprocedural cardiac multislice computed tomography (MSCT) and three-dimensional transoesophageal echocardiography (3DTOE). MSCT was performed using a Siemens Flash scanner (Siemens Healthcare GmbH, Erlangen, Germany), with images analysed retrospectively at 35% (systole) and 75% (diastole) of the cardiac cycle11. Real-time 3DTOE was performed using a Philips iE33 system (Philips Healthcare, Andover, MA, USA) with built-in Mitral Valve Quantification (MVQ) QLab 9 software. A combination of MSCT and 3DTOE was used to determine the D-shaped mitral annular area, perimeter, septal-to-lateral and intercommissural diameters, degree of mitral annular calcification, aorto-mitral angle, LV length, LVOT dimensions, and anterior mitral valve leaflet length (Figure 1). These data were used to select TMVR prosthesis size, identify the LV myocardial entry site, and assess the potential for LVOTO.
Figure 1. Preprocedural imaging. End-systolic MSCT frames were used to measure mitral annular dimensions in intracommissural (A) and anterioposterior (B) planes, and the aorto-mitral angle (C). As TMVI devices are large and may sit below the mitral annulus, an estimation of potential sub-mitral annular dimension was simulated at approximate LVOT depth (D). On 3DTOE imaging, LV length (apical epicardium to the mitral annulus) was measured in four conventional LV mid-oesophageal views (E-H). After confirming MR aetiology (I), LVOT dimensions in 2D and 3D (J & K) and the length of the anterior mitral valve leaflet were measured (L). Intracommissural and anterioposterior annular measurements were confirmed with X-plane (M) and 3DTOE (N-P) imaging.
THE TENDYNE TMVR SYSTEM AND PROCEDURAL DETAILS
Details of the Tendyne TMVR system and implantation procedure have been previously reported10. In brief, the Tendyne device is composed of a trileaflet porcine pericardial valve, sewn within two self-expanding nitinol stents, with an inner circular stent and an outer D-shaped stent to conform to the shape of the native mitral annulus (Figure 2). The size of the outer (sealing) frame ranges from 30 to 43 mm in the septal-lateral dimension and 34 to 50 mm in the intercommissural dimension. Implanted valves are selected to be larger than the native mitral orifice (extent of oversizing subject to ongoing evaluation)12. The cuff is raised along the straight aspect of the D shape, and this atrial flange is positioned in relation to the aortic-mitral continuity, thereby offering atrial sealing and anchoring. The tether stabilises the device, passing through the left ventricular wall where it is fixed to an epicardial pad. The device is implanted with the patient under general anaesthesia via a transapical approach through a small left mini-thoracotomy under continuous two-dimensional (2D) and 3DTOE visualisation (Figure 3). The LV access point for a coaxial approach to the mitral annular landing zone is determined by a combination of preprocedural MSCT planning (using the transection point of the mitral trajectory and LV epicardial surface)13 and periprocedural transthoracic echocardiography (visualising the true LV apex in relation to the aorta), and reconfirmed by the “finger test” (whereby the predetermined LV puncture site is reassessed on 3DTOE by visualising indentation of the predetermined LV access point by the surgeon’s finger in relation to the mitral trajectory) once the LV apex has been surgically exposed. Resheathing, repositioning, and retrieval of the prosthesis are possible until the final stage of deployment.

Figure 2. Tendyne device. Trileaflet porcine pericardial valve sewn within two self-expanding nitinol stents and an apical tethering/anchoring component (button-like pad and string tether).
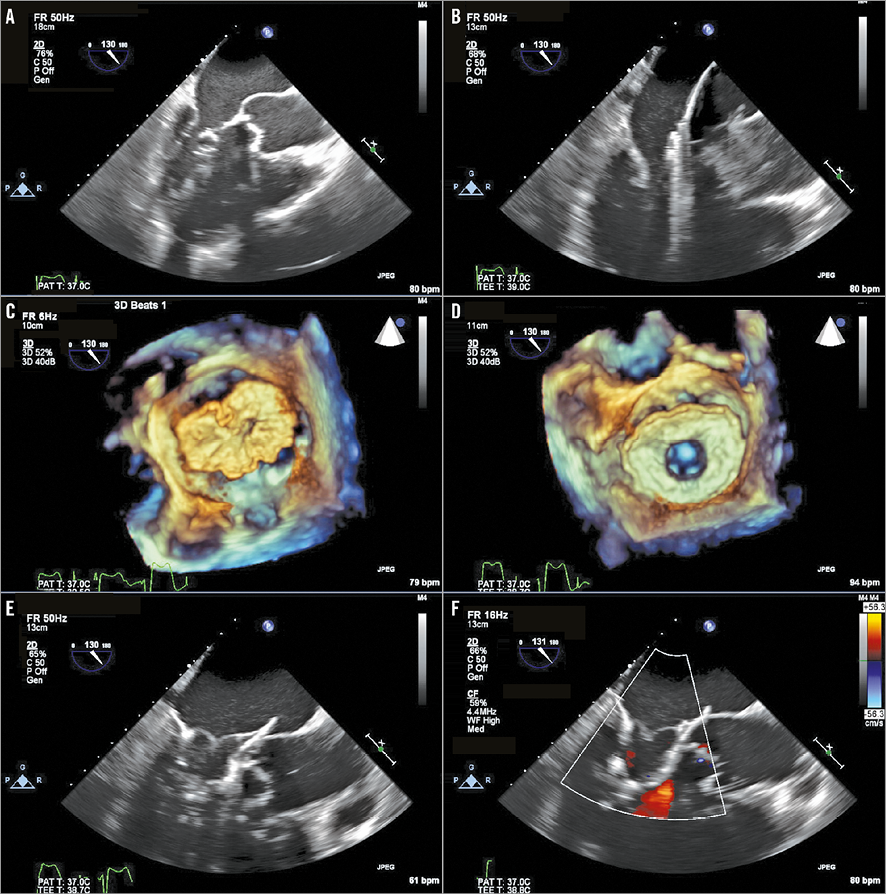
Figure 3. TMVR procedure. Balloon-tipped catheter tracked along guidewire and advanced into LA (A). Balloon is removed and 34 Fr sheath advanced over the wire into the LA (B). Tendyne delivery system extruded through the sheath in mid portion of LA and allowed to expand to 80% (C). Device rotated until correct anatomic position of D-shaped outer stent visualised (D). Device retracted into mitral annulus using gentle traction and fully deployed (E). Absence of transvalvular or paravalvular MR confirmed with colour Doppler (F).
ECHOCARDIOGRAPHY
Left ventricular end-diastolic dimensions (LVEDD) and end-systolic dimensions (LVESD), Simpson’s biplane left ventricular ejection fraction (LVEF), left ventricular stroke volume (LV-SV), and quantification of mitral and tricuspid regurgitation were measured according to established guidelines14,15. Specifically, LV-SV was measured as the difference between Simpson’s biplane end-diastolic volume and end-systolic volume.
STATISTICAL ANALYSIS
Numerical data are presented as mean±standard deviation and comparisons performed with the two-sample t-test using Stata statistical software, Version 10.1 (StataCorp, College Station, TX, USA).
Results
PERIPROCEDURAL AND 30 DAYS AFTER TMVR
No patient died and there were no VARC-216 complications (in particular, there were no cerebrovascular accidents, no vascular complications, and no bleeding events) in the periprocedural period and during the first 30 days after implantation. The first three patients reported marked symptomatic improvement immediately following TMVR; all were discharged to their own home independent in activities of daily living, after a mean length of hospital stay of 10 days. Patient #3 was discharged on the fifth postoperative day (Table 2).
Patient #4 (previous mitral repair with 38 mm Carpentier-Edwards Physio II ring) developed periprocedural LVOTO. A Heart Team discussion during the TMVR procedure concluded that the LVOTO was probably a dynamic sequela caused by systolic anterior motion of her relatively long anterior mitral valve leaflet in conjunction with a hypercontractile basal septum (preprocedural imaging suggested small LVOT diameter [1.7 cm], relatively long anterior mitral leaflet [2.75 cm], and an aorto-mitral angle of 118 degrees). As the patient had already declined redo open heart surgery, and since the Physio II ring was too large to accommodate any other CE-marked transcatheter device, we elected to retain the Tendyne device in situ and to monitor the LVOTO. On day 1 after TMVR however, the patient required mechanical support with an intra-aortic balloon pump (IABP), and subsequent venous-arterial extracorporeal membrane oxygenation (VA-ECMO). Five days after the TMVR procedure, a 22 mm CP Stent™ (NuMED, Hopkinton, NY, USA) was placed in the LVOT under fluoroscopic and 3DTOE guidance to alleviate LVOTO. 3DTOE confirmed excellent stent placement without compromise of the aortic valve function or compression of the LVOT stent due to LVOT contractility. LVOT peak gradient fell from 95 mmHg to 47 mmHg (Figure 4), and LV function improved sufficiently for the VA-ECMO and IABP support to be withdrawn after a further five and nine days, respectively. The patient had a prolonged hospital stay due to severe myopathy but, with intensive physiotherapy she made an excellent recovery, and was discharged home 65 days post TMVR with independence in activities of daily living. LVOT peak gradient reduced to 25 mmHg on pre-discharge transthoracic echo.
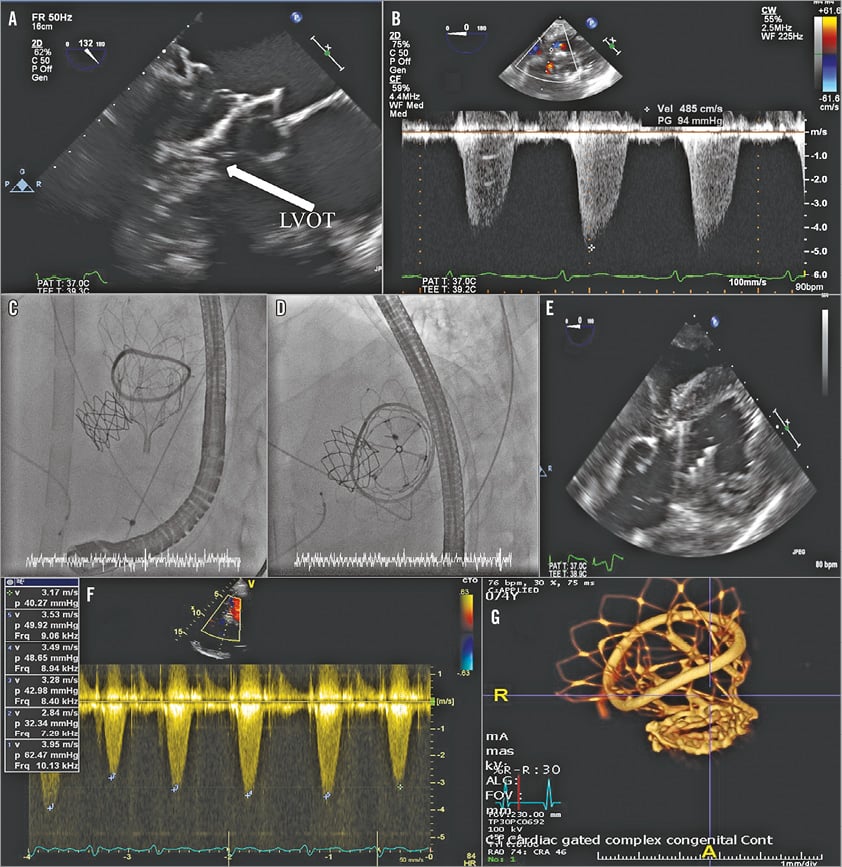
Figure 4. LVOTO and stenting. The combination of small LVOT dimension, long anterior mitral valve leaflet and immobile annuloplasty ring (A) resulted in significant LVOT gradient (B). 22 mm CP Stent placed under fluoroscopic (C & D) and TOE guidance (E) in the LVOT. The LVOT gradient decreased following LVOT stent deployment (F), and a stable position of the Tendyne device and CP Stent was confirmed on MSCT at six months (G).
30 DAYS
Transthoracic echocardiography (TTE) showed that no patient had transvalvular MR and none had significantly raised transmitral gradient (Table 2). Neither LV dimension nor LVEF changed (mean end-diastolic diameter [EDD] 6.0±0.7 cm at baseline to 6.0±0.6 cm; end-systolic diameter [ESD] 4.5±0.8 cm to 4.4±1.0 cm, LVEF 48±10% to 42±6%, p=0.11), but LV-SV increased (from 51±9 ml at baseline to 70±11 ml, p=0.02), and pulmonary artery systolic pressure (PASP) fell (from 61±25 mmHg to 38±25 mmHg, p=0.02). Patient #2 had biochemical haemolysis (low haptoglobin <0.3 g/L, elevated lactate dehydrogenase 4,431 IU/L, increased reticulocyte count 6.2%) and required a transfusion of two units of packed red blood cells on a single occasion; trivial PVL was noted on TTE. Tricuspid regurgitation (TR) severity decreased in all patients at 30 days, as did PASP (from 65±25 mmHg at baseline to 35±15 mmHg, p=0.003) (Figure 5).
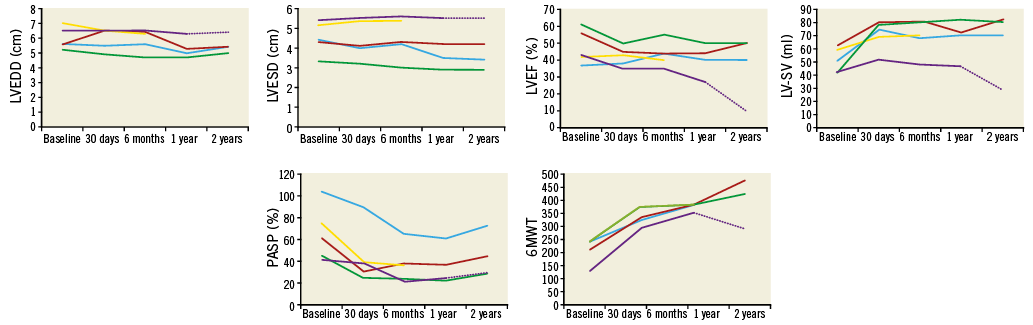
Figure 5. Effect of TMVR on LV cavity size, ejection fraction, stroke volume, pulmonary artery systolic pressure, and 6MWT distance. There was a non-significant reduction in LV cavity size, no change in LVEF, significant early followed by sustained increase in LV stroke volume and decrease in PASP, and progressive significant increase in 6MWT distance from baseline to two years.
SIX-MONTH OUTCOME AFTER TMVR
At six months, all patients were alive, living at home, and independent in activities of daily living. All reported continued symptomatic improvement with reduction in NYHA Class (from III to II), increased 6-minute walk test (6MWT) distance (from 205±51 m at baseline to 327±33 m, p=0.005) with continued reduction in TR severity and sustained reduction in PASP (to 37±17 mmHg) compared to 30 days. LV dimension, LVEF, and LV-SV remained unchanged compared to 30 days (mean EDD 5.9±0.8 cm, ESD 4.5±1.0 cm, LVEF 44±7%, LV-SV 70±13 ml). Patient #2 (with trivial PVL) developed paroxysmal atrial fibrillation (AF) and reported a possible transient ischaemic attack. A 3DTOE was performed which demonstrated no intra-cardiac thrombus. There appeared to be localised distortion of the Tendyne device posteromedially, causing a discrete localised PVL which appeared to “circumvent” the cuff of the device (Figure 6). However, MSCT demonstrated no device migration or stent fracture (Figure 6). Since the patient was clinically well (not anaemic), and closure of the PVL would have been technically challenging, we elected to manage his PVL conservatively, and he was commenced on warfarin for his paroxysmal AF. There were no new cases of LVOTO and, in the case of patient #4, the LVOT gradient fell further to 20 mmHg. A repeat MSCT in this patient demonstrated stable positions of both the Tendyne device and the CP stent, and no distortion of the apical tether site (Figure 4).
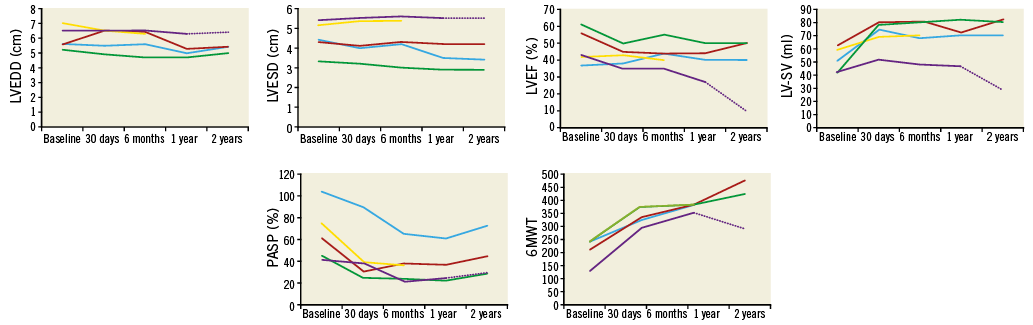
Figure 5. Effect of TMVR on LV cavity size, ejection fraction, stroke volume, pulmonary artery systolic pressure, and 6MWT distance. There was a non-significant reduction in LV cavity size, no change in LVEF, significant early followed by sustained increase in LV stroke volume and decrease in PASP, and progressive significant increase in 6MWT distance from baseline to two years.
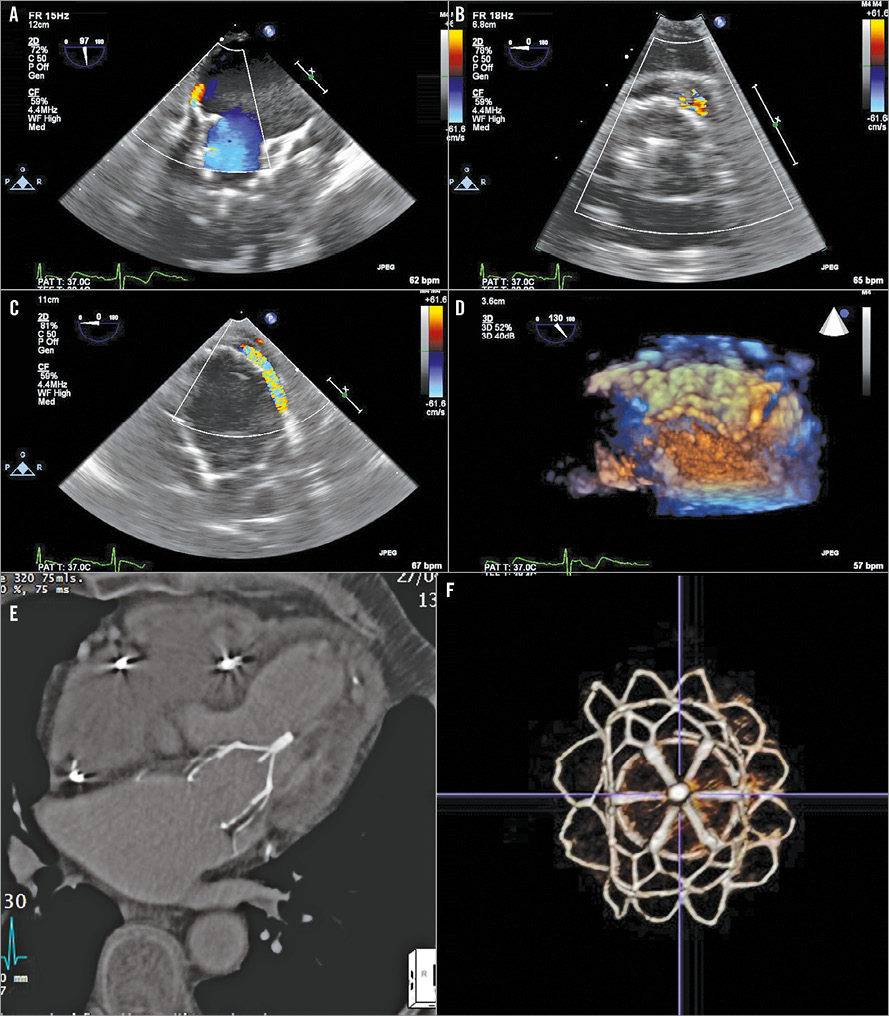
Figure 6. Paravalvular leak after TMVR. A posteromedial (A & B) discrete PVL on TOE, which appeared to “circumvent” the cuff of the device (C). Although 3DTOE appeared to show localised distortion of the Tendyne device posteromedially (D), MSCT confirmed no device migration or distortion of the LV apical tether site (E) or stent fracture of the Tendyne device (F).
ONE-YEAR OUTCOME AFTER TMVR
Four patients were alive 12 months after TMVR. Patient #3 (who had been in atrial fibrillation and intermittently non-compliant with antithrombotic medication before the TMVR procedure) died nine months after TMVR following a cerebrovascular accident. TTE performed two days before his death demonstrated normal device function with no evidence of thrombus; there was no opportunity to perform an MSCT due to the short time between clinical presentation and death. Patient #5 was admitted for an atrial fibrillation ablation, for which his warfarin had been temporarily discontinued for five days immediately prior to ablation. Preprocedural TTE documented several small highly mobile masses attached to the device stent (but not on the Tendyne leaflets), which were highly suggestive of thrombus (Figure 7). The ablation procedure was postponed, and he was recommenced on therapeutic dose warfarin. Repeat TTE and MSCT one month later showed no evidence of leaflet thickening or thrombus, and no distortion of the LV apical tether site.
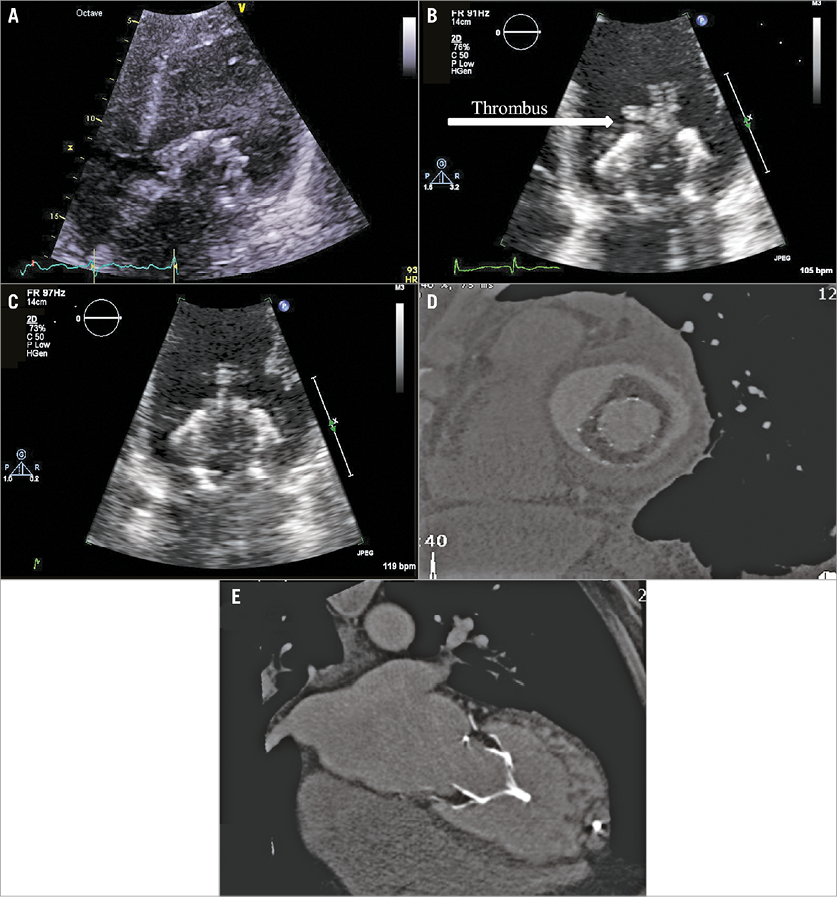
Figure 7. Thrombus development on the TMVR stent. Compared to 30 days (A), several highly mobile masses attached to the Tendyne device at nine months (B). No evidence of thrombus on either TTE (C) or MSCT (D) tomography one month after re-institution of warfarin. MSCT confirmed no distortion of the LV apical tether site (E).
18-MONTH TO TWO-YEAR OUTCOME AFTER TMVR
All alive patients reported sustained symptomatic improvement and reduction in NYHA class (Table 2). TTE demonstrated valve stability with no late migration, no evidence of transvalvular MR or new PVL, and sustained reductions in both TR severity and PASP (38±20 mmHg). LVEF and LV-SV decreased in patient #5 (to 10% and 30 ml, respectively), in whom 6MWT had also decreased (Figure 5). Overall, however, there was no difference in LV dimensions, LVEF, or LV-SV as a group compared to six months (EDD 5.5±0.6 cm, ESD 4.0±1.1 cm, LVEF 38±19%, LV-SV 65±24 ml). The early PVL in patient #2 remained trivial with no clinical evidence of haemolysis, and the LVOT peak gradient in patient #4 remained low (at 20 mmHg).
Discussion
Our first-in-human cases for compassionate use in five high-risk patients with intractable heart failure and severe MR demonstrate the feasibility of Tendyne TMVR. No patients died during the periprocedural period. The single death that occurred was in a patient who had been non-compliant with antithrombotic medication and who sustained a cerebrovascular accident nine months after TMVR. TTE performed two days prior to his death reported no evidence of device degeneration or thrombus formation. There was no opportunity for an MSCT so we were unable to exclude leaflet thickening or device thrombosis completely in this patient. However, three of the four remaining patients underwent MSCT scans 6-12 months following TMVR, and none demonstrated leaflet thickening, device thrombosis, or distortion of the apex due to overtensioning of the apical tether. Our fifth patient, however, did develop multiple thrombi on the Tendyne stent (though not on the leaflets) during a temporary cessation of warfarin, which resolved on recommencing therapeutic anticoagulation. Thus, although evidence-based guidance on anticoagulation is lacking in this field, it would seem prudent to continue long-term therapeutic anticoagulation (target INR 3-4) in these patients.
Four out of five patients still alive 18 months/two years after TMVR reported significant and sustained improvement in symptomatic status. Moreover, TTE demonstrated valve stability with complete elimination of transvalvular MR. Immediate elimination of transvalvular MR with preservation of LVEF in the patient group as a whole was associated with an immediate then sustained increase in mean LV forward stroke volume. Furthermore, at two years there was no increase in transvalvular gradient, and significant sustained reductions in the severity of TR and PASP in all patients. However, it is widely recognised that correction of secondary MR may not prevent progressive LV disease and dysfunction in some patients, and we noted that elimination of transvalvular MR was accompanied by progressive reduction in LVEF and stroke volume in patient #5.
A concern of the Tendyne device had been the effect of LV remodelling on the tether tension with time, but, despite small (non-significant) reductions in LV size, no patient demonstrated device migration on follow-up TTE. Only one patient had PVL, which was detected early after implantation. Possible explanations include device migration (but there was no evidence of this on TOE or MSCT), localised fracture of the Tendyne stent (but there was no evidence of this on MSCT), or the PVL may have been present at the time of implantation, but missed on post-procedural check imaging as it was so small. In any case, this patient’s PVL and severity of haemolysis both decreased at two years with conservative management. No patient developed late PVL.
No patient with native mitral valve annulus developed periprocedural or late LVOTO. However, patient #4 had a mitral annuloplasty ring, and had (on reflection) a small LVOT diameter, a relatively long native anterior mitral valve leaflet, and a relatively narrow aorto-mitral angle. We believe the combination of all four factors led to this patient developing dynamic periprocedural LVOTO. Although the device could have been removed during the procedure (a design feature), there were limited alternative treatments available as this patient had refused repeat surgical intervention. Instead, we elected to stent the LVOT to relieve the LVOTO. We inflated a 20 mm BiB® balloon (NuMED) to interrogate the outflow tract anatomy and its compliance. This demonstrated good stent apposition between the LVOT wall and Tendyne device frame. We elected to mount a 22 mm CP Stent on the balloon which was deployed successfully, and which resulted in a stent position which covered the most significantly obstructed segment of the LVOT, producing an immediate reduction in the pressure gradient across the LVOT. We felt that the reduction in LVOT peak gradient to 20 mmHg pre-discharge, the continued reduction in LVOT peak pressure gradient two years after TMVR, and the fact that the patient clinically improved (6MWT increased from 240 m before TMVR to 420 m at two years) lend weight to the initial diagnosis of dynamic LVOTO at the time of periprocedural LVOTO in this patient.
Study limitations
Although the number in our study is small, it is the only long-term Tendyne study to date. In our patient group, TMVR using a Tendyne device was performed on compassionate grounds, in patients in whom a follow-up MSCT was not mandated. Nevertheless, we were able to perform MSCT on three out of our five patients on clinical grounds; in these 3 patients, leaflet thickening, device thrombosis, and distortion of the apex due to overtensioning of the apical tether were not present. An ongoing early feasibility study and continuing follow-up of the treated patients will further define the safety and efficacy of this procedure.
Conclusions
Transcatheter mitral valve replacement is a less invasive, technically feasible alternative for patients with severe mitral regurgitation for whom the risks associated with surgical valve repair or replacement are prohibitive. In long-term follow-up after the compassionate use of Tendyne TMVR in a high-risk surgical group, the majority of patients were alive, there was sustained improvement in symptoms, and the device was stable with good haemodynamic function on echocardiography. The first-in-man experience with the Tendyne device thus provides a promising beginning. However, even in our small study, we experienced recognised complications associated with TMVR, namely LV outflow tract obstruction, paravalvular leak with haemolysis, and device thrombosis. Early feasibility studies and continued follow-up of patients treated with Tendyne TMVR are required to describe further the safety and efficacy of this catheter-based mitral valve implantation technique and its role in the treatment of valvular heart disease.
| Impact on daily practice Transcatheter mitral valve intervention for high-risk surgical patients is emerging. Since almost half of patients with moderate to severe mitral regurgitation are not referred for conventional mitral valve surgery on the basis of advanced age or multiple comorbidities, transcatheter mitral valve replacement (TMVR) is beginning to materialise in the footsteps of the success of transcatheter aortic valve implantation for patients with severe aortic stenosis at high surgical risk. Our team has previously reported favourable periprocedural and 30-day outcomes in first-in-man compassionate use of the TMVR Tendyne device. We now show that the majority of these high-risk patients are alive, feel better, and have stable device haemodynamic function in the long term. The first-in-man experience with the Tendyne device thus provides a promising beginning at the dawn of TMVR. |
Acknowledgements
The authors wish to thank Georg Lutter, Lucian Lozonschi, Eduardo de Mar, Dan Mans, Bob Vilund, Jeff Franco, and Jessica Kleine (and their colleagues at Tendyne) for contributions made to the development of the Tendyne system.
Conflict of interest statement
N. Moat is a consultant for Medtronic and has received speaker’s fees from Abbott, Medtronic, and Edwards. The other authors have no conflicts of interest to declare.

